Figure 2. Astrocytic contribution to the mechanisms underlying temporal lobe and absence epilepsy.
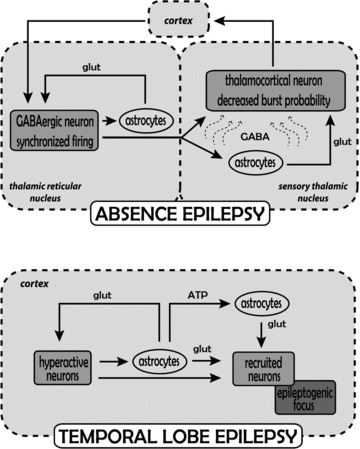
Absence epilepsy: the generation of absence seizures involves abnormal firing activity among cortical cells and neurons in the thalamic reticular and sensory thalamic nuclei. During absence seizures increased firing in GABAergic neurons of the thalamic reticular nucleus (which is driven by the synchronized cortical input) leads to enhanced GABA levels (curved arrows) in sensory thalamic nuclei and the resulting increase in tonic GABAA current in thalamocortical neurons since GAT-1 transporter function is diminished (see Fig. 1). The increased tonic GABAA current reduces, but does not abolish, firing in the thalamocortical neurons, while at the same time blocks faithful transmission of sensory inputs and reduces the effect of any potential increase in astrocytically released glutamate on these neurons. On the other hand, the putative increase in astrocytically released glutamate re-enforces synchronized firing in reticular thalamic neurons. It is unlikely that any increased astrocytically released GABA affects reticular thalamic neurons since they show no tonic GABAA current. It remains to be determined whether similar alterations occur in the cortical territory. Temporal lobe epilepsy: the generation of temporal lobe seizures involves abnormal firing activity initially restricted to a group of cortical neurons. Increased glutamate release activates local astrocytes, which by releasing glutamate signal back to neurons, thus enhancing their hyperactivity, and also signal to other astrocytes and neurons in nearby regions increasing the probability of neuronal recruitment into the epileptogenic focus.
