Abstract
Reduced bactericidal efficacy at a high inoculum is known as the inoculum effect (IE). We used neutropenic mice to compare the IEs of ceftobiprole (CFB), daptomycin (DAP), linezolid (LZD), and vancomycin (VAN) against 6 to 9 strains of Staphylococcus aureus and 4 strains of Streptococcus pneumoniae at 2 inocula in opposite thighs of the same mice. Neutropenic mice had 104.5 to 105.7 CFU/thigh (low inoculum [LI]) in one thigh and 106.4 to 107.2 CFU/thigh (high inoculum [HI]) in the opposite thigh when treated for 24 h with subcutaneous (s.c.) doses every 12 h of DAP at 0.024 to 100 mg/kg of body weight and LZD at 0.313 to 320 mg/kg and s.c. doses every 6 h of CFB at 0.003 to 160 mg/kg and VAN at 0.049 to 800 mg/kg. Dose-response data were analyzed by a maximum effect (Emax) model using nonlinear regression. Static doses for each drug and at each inoculum were calculated, and the difference between HI and LI (IE index) gave the magnitude of IE for each drug-organism combination. Mean (range) IE indexes of S. aureus were 2.9 (1.7 to 4.6) for CFB, 4.1 (2.6 to 9.3) for DAP, 4.6 (1.7 to 7.1) for LZD, and 10.1 (6.3 to 20.3) for VAN. In S. pneumoniae, the IE indexes were 2.5 (1.3 to 3.3) for CFB, 2.0 (1.6 to 2.8) for DAP, 1.9 (1.7 to 2.2) for LZD, and 1.5 (0.8 to 3.2) for VAN; these values were similar for all drugs. In S. aureus, the IE was much larger with VAN than with CFB, DAM, and LZD (P < 0.05). An in vivo time course of vancomycin activity showed initiation of killing at 4- to 16-fold-higher doses at HI than at LI despite similar initial growth of controls.
INTRODUCTION
Staphylococcus aureus and Streptococcus pneumoniae are both important Gram-positive pathogens in many clinical situations, such as sepsis, pneumonia, and infective endocarditis (1, 2). Therefore, it is essential to choose the correct antibiotics if treatment is to be successful, and clinicians usually regard in vitro susceptibility testing, pharmacodynamic characteristics, and prior clinical trials as guides to antibiotic choice (3–6). However, in many in vivo situations, patients would be different from the highly standardized methods used in the microbiology laboratory. For example, in infections such as endocarditis, osteomyelitis, and pneumonia, the infecting inoculum can be much higher than that used for in vitro susceptibility testing. This can result in reduced bactericidal efficacy or even clinical failure (7–9).
Over 60 years ago, Woods first described this “inoculum effect” (IE) in vitro (10). This effect has been observed mainly in S. aureus exposed to β-lactams and glycopeptides, such as vancomycin, but usually at inocula higher than 107 bacteria per ml (11–13). We recently have reported a high dosage requirement for vancomycin with multiple strains of methicillin-resistant S. aureus (MRSA) in a murine thigh infection model at inocula of 106 to 107 CFU/thigh. Ten-fold-lower inocula showed much lower dosage requirements for stasis and 1-log kill (14).
In this study, we compare the inoculum effects in vivo against methicillin-susceptible and -resistant S. aureus (MSSA and MRSA, respectively) and penicillin-susceptible and -resistant S. pneumoniae (PSSP and PRSP, respectively) with ceftobiprole (CFB), daptomycin (DAP), linezolid (LZD), and vancomycin (VAN). Two different inocula injected into opposite thighs of the same neutropenic mice facilitate an accurate comparison of the inoculum effects with various antibiotics and different organisms. We have also studied the time course of antimicrobial activity for different doses of vancomycin against high and low inocula of 2 strains of MRSA injected into opposite thighs of the same neutropenic mice.
(These studies were presented in part at the 47th and 48th Interscience Conferences on Antimicrobial Agents and Chemotherapy, Chicago, IL, September 2007, and Washington, DC, October 2008.)
MATERIALS AND METHODS
Organisms, media, and antibiotics.
Two methicillin-susceptible S. aureus (MSSA) strains, four to seven MRSA (including 2 heteroresistant vancomycin-intermediate S. aureus [VISA]) strains, two penicillin-susceptible S. pneumoniae (PSSP) strains, and two penicillin-resistant S. pneumoniae (PRSP) strains were used for these experiments. Both of the heteroresistant VISA strains had population analysis profile (PAP) areas under the concentration-time curve (AUCs) greater than the value for the reference strain MRSA Mu3 (15). All the other MRSA strains had much lower PAP AUCs than the reference strain.
S. aureus and S. pneumoniae organisms were grown, subcultured, and quantified in Mueller-Hinton broth (Difco Laboratories, Detroit, MI), on Mueller-Hinton agar (Difco), and on sheep blood agar plates (Remel, Milwaukee, WI). The lower limit of organism quantification in these studies was 100 CFU/thigh or ml.
Ceftobiprole (Johnson and Johnson, Rariton, NJ), daptomycin (Cubist Pharmaceuticals, Inc., Lexington, MA), and linezolid (Pfizer Inc., New York, NY) were supplied by each company. Oxacillin and penicillin were purchased from Sigma Chemical (St. Louis, MO). VAN was provided by Hospira, Inc. (Lake Forest, IL). Stock solutions of each antibiotic were freshly prepared at the beginning of each experiment and kept frozen at −4°C.
In vitro susceptibility tests.
The MICs of CFB, DAP, LZD, VAN, penicillin, and oxacillin for the various isolates were determined by using standard CLSI microdilution methods (16). The broth microdilution wells were read at 20 h after incubation at 35°C.
Murine infection model.
Six-week-old specific-pathogen-free, female ICR/Swiss mice weighing 23 to 27 g (Harlan Sprague-Dawley, Madison, WI) were used for all studies. The animals used were maintained in accordance with the criteria of the American Association for Accreditation of Laboratory Animal Care (17). All studies were approved by the Animal Research Committee of the William S. Middleton Memorial Veterans Affairs Hospital. The mice were made neutropenic (polymorphonuclear cell count, <100 mm3) by two intraperitoneal (i.p.) injections of cyclophosphamide (Bristol-Myers Squibb, Princeton, NJ) at 150 mg/kg of body weight at 4 days and 100 mg/kg at 1 day before infection. Broth cultures of freshly plated bacteria were grown to logarithmic phase overnight to an absorbance at 580 nm of 0.3 (Spectronic 88; Bausch & Lomb, Rochester, NY). After dilution into fresh Mueller-Hinton broth, thigh infections with each of the isolates and inocula were produced by injection of 0.1 ml of each inoculum into the thighs of isoflurane-anesthetized (Baxter Healthcare Corporation, Deerfield, IL) mice at 2 h before therapy with antibiotics. The starting inocula obtained at 2 h were 104.5 to 105.7 CFU/thigh (low inoculum [LI]) in one thigh and 106.4 to 107.2 CFU/thigh (high inoculum [HI]) in the opposite thigh simultaneously.
At 24 h after the start of antibiotics, the animals were killed by CO2 asphyxiation. The thighs were immediately removed, homogenized, and prepared as 10% homogenates in 0.9% sterile iced saline. Viable counts were determined by plating duplicate 10-μl aliquots of 10-fold serially diluted samples of homogenate in saline on Mueller-Hinton agar for S. aureus and on sheep blood agar for S. pneumoniae. Data were expressed as the mean (± standard deviation) log10 CFU/thigh (18).
Vancomycin pharmacokinetics.
Single-dose serum pharmacokinetic studies of VAN were performed in neutropenic thigh-infected mice given subcutaneous (s.c.) doses (0.2 ml/dose) of VAN at 25, 50, 100, 200, and 800 mg/kg. For each of the doses examined, 4 groups of 3 mice were sampled by retro-orbital puncture at 0.25- to 1-h intervals over 6 h (sample times included 0.25, 0.5, 0.75, 1, 1.5, 2, 3, 4, 5, and 6 h). Individual animals were sampled 3 or 4 times. Serum VAN concentrations were determined by standard microbiologic assays using Bacillus subtilis ATCC 6633 as the test organism. The lower limit of detection was 1.0 μg/ml. The zone sizes were linear from 1 to 64 μg/ml; higher concentrations were determined in 1:4 dilutions of individual mouse serum in pooled mouse serum. The intraday variation was less than 10%. Protein binding in the serum of infected neutropenic mice was performed by ultrafiltration at concentrations of 10 and 50 μg/ml (19). Pharmacokinetic parameters were calculated by noncompartmental analysis. The AUC was calculated from the mean concentrations by the trapezoidal rule. For doses for which no kinetics were determined, pharmacokinetic parameters were interpolated from the values of the nearest studied doses.
Treatment protocols.
Mice were treated for 24 h with doses every 12 hours of DAP at 0.024 to 100 mg/kg and LZD at 0.313 to 320 mg/kg and doses every 6 hours of CFB at 0.003 to 160 mg/kg and VAN at 0.049 to 800 mg/kg. Drugs were administered s.c. in 0.2-ml volumes. Saline-treated control mice were killed just before treatment and after 24 h. Three mice were used for each time point and dosage regimen for each organism.
A second study examined the time course of vancomycin activity against HI and LI of two strains of MRSA (19936 and 11888) injected into opposite thighs of the same mice. Starting inocula were 104.93 to 105.03 CFU/thigh (LI) in one thigh and 106.80 to 106.85 CFU/thigh (HI) in the opposite thigh. The animals were treated for 24 h with s.c. doses every 6 hours of VAN at 3.125, 12.5, 50, and 200 mg/kg. Groups of three mice were sacrificed at 2, 4, 6, 8, 12, 14, 18, and 24 h; saline-treated controls were killed at 0, 2, 6, 12, and 24 h. Thighs were removed and processed as outlined above.
In vitro kill curves.
The effects of different inocula on the in vitro killing of MRSA 19936 and MRSA 11888 at increasing concentrations of VAN were compared in MH broth. Both strains of MRSA at HI (107 CFU/ml) and LI (105 CFU/ml) were exposed for 24 h to VAN at 1, 2, 4, and 8 times the MIC. Samples were removed at 0, 1, 2, 3, 4, 5, 6, 12, and 24 h. Aliquots of serial 10-fold dilutions were plated on agar for CFU determinations.
Data analysis.
The results of these studies were analyzed by using a sigmoid dose-effect model (20–22). The model is derived from the Hill equation E = (Emax × DN)/(ED50N + DN), where E is the effect or, in this case, the log change in CFU/thigh between treated mice and untreated controls after the 24-h period of study, Emax is the maximum effect, D is the 24-h total dose, ED50 is the dose required to achieve 50% of Emax, and N is the slope of the dose-effect curve. The parameters of Emax, ED50, and N were estimated by using nonlinear least-squares regression (Sigma Stat version 3.10; Systat Software, San Jose, CA). Static doses for each drug and at each inoculum were calculated and compared by t tests. The ratio of the static dose between HI and LI (IE index) gave the magnitude of the IE for each drug-organism combination, and these were compared by t tests and one-way analysis of variance with Duncan's test (Sigma Stat).
The correlations between efficacies at high and low inocula and time above the MIC for CFB and ratios of free drug AUC from 0 to 24 h (fAUC0-24) to MIC for DAP, LZD, and VAN were also determined by nonlinear least-squares regression (Sigma Stat). The coefficient of determination, or R2, was used to estimate the percentage of variance in efficacy that can be attributed to regression with the important pharmacokinetic (PK)/pharmacodynamic (PD) indices. Values for the static and 1-log kill doses were determined at both LI and HI for staphylococci (6 to 9 strains) and pneumococci (4 strains).
In vivo and in vitro killing and growth rates (change in log10 CFU/thigh/h) for VAN were determined over 24 h and compared for each strain of MRSA at LI and HI. All the data at each time point from 0 to 12 h for untreated controls and 0 to 24 h for vancomycin-treated animals or cultures were used with linear regression to determine killing and growth rates.
RESULTS
In vitro susceptibility testing.
The study organisms and the MICs of CFB, DAP, LZD, VAN, oxacillin, and penicillin are listed in Table 1. MICs of CFB against S. pneumoniae strains varied by 32-fold. The ranges of MICs for the other three drugs against S. pneumoniae and all four drugs against S. aureus varied 4-fold or less. The in vitro potency of CFB against S. aureus was modestly affected by β-lactam resistance.
Table 1.
In vitro activity of antimicrobials against strains of S. aureus and S. pneumoniae
| Organisma | MIC (mg/liter) |
|||||
|---|---|---|---|---|---|---|
| Ceftobiprole | Daptomycin | Linezolid | Vancomycin | Oxacillin | Penicillin | |
| S. aureus strains | ||||||
| ATCC 29213 (MSSA) | 0.5 | 0.5 | 2 | 1.0 | 0.12 | |
| SMITH (MSSA) | 0.5 | 0.25 | 4 | 0.5 | 0.06 | |
| 22115 (MRSA) | 1 | 0.25 | 2 | 1.0 | >64 | |
| 18000 (MRSA) | 1 | 0.25 | 2 | 0.5 | 16 | |
| 12248 (MRSA) | 2 | 0.12 | 2 | 0.5 | 64 | |
| 11888 (MRSA) | 2 | 0.25 | 1 | 0.5 | >64 | |
| 19936 (MRSA) | 0.5 | >64 | ||||
| 21773 (hVISA) | 0.5 | 64 | ||||
| 20594 (hVISA) | 0.5 | >64 | ||||
| S. pneumoniae strains | ||||||
| ATCC 10813 (PSSP) | 0.03 | 0.12 | 1 | 0.12 | < 0.008 | |
| MNO-418 (PSSP) | 0.015 | 0.25 | 0.5 | 0.12 | < 0.008 | |
| CDC 1293 (PRSP) | 0.5 | 0.25 | 0.5 | 0.25 | 2.0 | |
| CDC 1329 (PRSP) | 0.25 | 0.12 | 0.25 | 0.12 | 2.0 | |
Abbreviations: MSSA, methicillin-susceptible S. aureus; MRSA, methicillin-resistant S. aureus; hVISA, heteroresistant vancomycin-intermediate S. aureus; PSSP, penicillin-susceptible S. pneumoniae; PRSP, penicillin-resistant S. pneumoniae.
Pharmacokinetics of vancomycin and other drugs.
The PK characteristics of VAN in infected neutropenic mice following a single subcutaneous dose of 25, 50, 100, 200, or 800 mg/kg VAN are shown in Table 2. Over the dosage range studied, PK parameters were moderately dose dependent, with the elimination half-life increasing 2-fold, from 26.5 min to 52 min, as the dose rose from 25 to 800 mg/kg. The AUC/dose values for the escalating single doses ranged from 0.83 to 1.1, and the maximum concentration of the drug (Cmax)/dose values ranged from 0.32 to 1.4. Protein binding of VAN in mouse serum was 26%.
Table 2.
Single-dose pharmacokinetics of vancomycin in infected micea
| Dose (mg/kg) | Cmax (mg/liter) | t1/2 (min) | AUC (mg · h/liter) |
|---|---|---|---|
| 25 | 34.1 | 26.5 | 27.6 |
| 50 | 57.5 | 29.0 | 45.0 |
| 100 | 72.7 | 34.3 | 85.8 |
| 200 | 112 | 42.0 | 165 |
| 800 | 252 | 52.0 | 680 |
t1/2, half-life.
The PK characteristics for DAP, LZD, and CFB were taken from our recent publications using the same neutropenic thigh model (20–22). The AUCs for single s.c. doses of DAP at 10 and 40 mg/kg were 93 and 375 mg · h/liter, respectively. The AUCs for LZD at s.c. doses of 20 and 80 mg/kg were 18.5 and 112 mg · h/liter, respectively. For CFB, the PK characteristics were slightly dose dependent at doses of 10, 40, and 160 mg/kg. The half-life increased from 19.0 to 31.7 min, while the AUC/dose increased from 0.6 to 1.3 and the Cmax/dose decreased slightly from 1.1 to 0.9. The protein binding in mouse serum was 90% for DAP, 22% for LZD, and <5% for CFB.
Twenty-four-hour static dose determinations according to inoculum size.
At the start of therapy, the difference in inocula between the thighs in each mouse was 1.8 ± 0.3 log10 CFU/thigh, with no significant differences according to the organisms and presence of resistance. Strains grew well in each thigh (1.1 to 4.1 and 0.8 to 2.8 log10 CFU/thigh in LI and HI, respectively, over 24 h in controls).
Calculations of the doses (mg/kg/24 h) of CFB, DAP, LZN, and VAN required to achieve stasis at LI and HI against multiple organisms are shown in Table 3. Mean (range) IE indexes for S. aureus were 2.9 (1.7 to 4.6) for CFB, 4.1 (2.3 to 9.3) for DAP, 4.6 (1.7 to 7.1) for LZD, and 10.1 (6.3 to 20.3) for VAN. The median IE indexes for CFB, DAP, LZD, and VAN were 2.8, 3.1, 4.1, and 7.4, respectively. Methicillin resistance did not alter the magnitude of static doses required for efficacy for all four study drugs. The IE index of VAN for S. aureus was higher than those for the other three antibiotics (P < 0.002). One strain (MRSA 11888) had the highest IE indexes for DAP, LZD, and VAN, which increased the mean values for those three drugs.
Table 3.
Inoculum effect and index for each drug and each organisma
| Organism | Static dose (mg/kg/day) and IE index with: |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ceftobiprole |
Daptomycin |
Linezolid |
Vancomycin |
|||||||||
| LI | HI | IE index | LI | HI | IE index | LI | HI | IE index | LI | HI | IE index | |
| MSSA | ||||||||||||
| 29213 | 2.54 | 11.8 | 4.6 | 20.8 | 53.6 | 2.6 | 100 | 323 | 3.2 | 34.4 | 243 | 7.1 |
| SMITH | 4.51 | 8.55 | 1.9 | 5.61 | 13.6 | 2.4 | 63.1 | 316 | 5.0 | 12.1 | 75.8 | 6.3 |
| MRSA | ||||||||||||
| 22115 | 15.3 | 57.0 | 3.7 | 6.25 | 14.1 | 2.3 | 56.5 | 222 | 3.9 | 16.9 | 125 | 7.4 |
| 18000 | 4.41 | 7.50 | 1.7 | 2.49 | 11.3 | 4.5 | 51.7 | 212 | 4.3 | 7.52 | 112 | 14.9 |
| 12248 | 50.3 | 97.5 | 1.9 | 2.93 | 10.4 | 3.6 | 94.2 | 162 | 1.7 | 9.40 | 95.8 | 10.2 |
| 11888 | 40.0 | 149 | 3.7 | 1.70 | 15.8 | 9.3 | 8.81 | 62.5 | 7.1 | 12.7 | 258 | 20.3 |
| 19936 | 6.01 | 66.4 | 11.0 | |||||||||
| 21773 | 13.9 | 92.4 | 6.6 | |||||||||
| 20594 | 8.16 | 58.4 | 7.2 | |||||||||
| S. aureus (mean ± SD) | 2.9 ± 1.2 | 4.1 ± 2.7 | 4.6 ± 2.3 | 10.1 ± 4.7b | ||||||||
| PSSP | ||||||||||||
| 10813 | 0.182 | 0.237 | 1.3 | 0.304 | 0.553 | 1.8 | 29.8 | 65.4 | 2.2 | 1.10 | 3.48 | 3.2 |
| MNO-418 | 0.134 | 0.358 | 2.7 | 1.02 | 2.01 | 2.0 | 28.3 | 48.2 | 1.7 | 2.95 | 3.45 | 1.2 |
| PRSP | ||||||||||||
| CDC 1293 | 4.04 | 11.1 | 2.7 | 2.08 | 5.77 | 2.8 | 17.3 | 34.7 | 2.0 | 8.07 | 6.08 | 0.8 |
| CDC 1329 | 2.56 | 8.37 | 3.3 | 0.551 | 0.890 | 1.6 | 9.30 | 17.0 | 1.8 | 2.52 | 2.37 | 0.9 |
| S. pneumoniae (mean ± SD) | 2.5 ± 0.8 | 2.0 ± 0.5 | 1.9 ± 0.2c | 1.5 ± 1.1c | ||||||||
Abbreviations: LI, low inoculum; HI, high inoculum; IE, inoculum effect; MSSA, methicillin-susceptible S. aureus; MRSA, methicillin-resistant S. aureus; PSSP, penicillin-susceptible S. pneumoniae; PRSP, penicillin-resistant S. pneumoniae; SD, standard deviation.
Statistically different from the IE indexes of ceftobiprole, daptomycin, and linezolid against Staphylococcus aureus (P < 0.05).
Statistically less for S. pneumoniae than for S. aureus (P < 0.05).
In S. pneumoniae, the mean (range) IE indexes were 2.5 (1.3 to 3.3) for CFB, 2.0 (1.6 to 2.8) for DAP, 1.9 (1.7 to 2.2) for LZD, and 1.5 (0.8 to 3.2) for VAN. The median IE indexes for CFB, DAP, LZD, and VAN were very similar, at 2.7, 1.9, 1.9, and 1.1, respectively. Penicillin resistance raised the magnitude of the static dose for CFB but not for the other three antibiotics. The IE indexes for all four drugs were not statistically different (P = 0.38). The IE index of VAN for S. pneumoniae was lower than that for S. aureus (P = 0.005). The IE index of LZD for S. pneumoniae was also lower than the values for S. aureus (P = 0.04). The values for the other two drugs for S. pneumoniae were not statistically different from that for S. aureus.
Pharmacodynamic analysis and comparison according to the inoculum size.
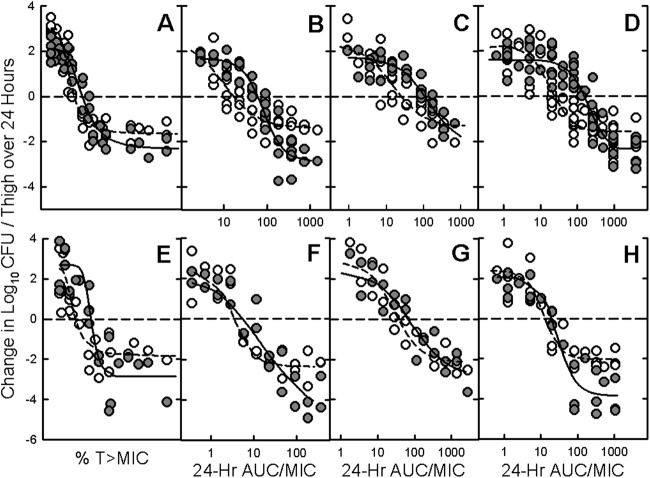
The relationships between time above the MIC for CFB and fAUC0-24/MIC for DAP, LZD, and VAN at HI and LI are illustrated in Fig. 1 for all 6 to 9 staphylococci and 4 pneumococci. Coefficients of determination (R2) at different inocula were highly statistically significant for all drug-organism combinations and ranged from 67 to 93% with CFB, 76 to 91% with DAP, 77 to 89% with LZD, and 81 to 88% with VAN. Surprisingly, the bactericidal activities of DAP and VAN were reduced at LI compared to the activities at HI.
Fig 1.
Relationship between time above the MIC for ceftobiprole and 24-hour AUC/MIC ratios for daptomycin, linezolid, and vancomycin, and change in log10 CFU/thigh at both low (open circles) and high (solid circles) inocula against Staphylococcus aureus (MSSA and MSSA) and Streptococcus pneumoniae (PSSP and PRSP) that were injected into opposite thighs of neutropenic mice. Each symbol represents the mean of the data for 3 mice. The dotted line is the line of best fit for low-inoculum data, and the solid line is the line of best fit for high-inoculum data.
The calculated times above the MIC for CFB and the total and free (f) drug AUC0-24/MIC values for DAP, LZD, and VAN for stasis and 1-log kill with staphylococci and pneumococci at LI and HI are shown in Table 4. The times above the MIC for CFB for stasis and 1-log kill were low and ranged from 8 to 20% of the dosing interval at both LI and HI. The fAUC0-24/MIC values with DAP, LZD, and VAN for stasis with both organisms at LI ranged from 5 to 30. The value for these drugs at HI increased at most 2-fold for S. pneumoniae. However, the fAUC0-24/MIC values at HI for S. aureus were 3.6-fold higher for DAP, 4.3-fold higher for LZD, and 6.4-fold higher for VAN than the values obtained at LI for the same drugs.
Table 4.
Pharmacokinetic-pharmacodynamic indexes and the magnitudes (± standard errors) for free and total drug required for stasis and 1-log10 kill at 24 h of S. aureus and S. pneumoniae at low and high inocula in opposite thighs of the same neutropenic mice
| Organism | Inoculum | Time above the MIC (%) with ceftobiprole |
f AUC/MIC (total AUC/MIC) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Daptomycin |
Linezolid |
Vancomycin |
|||||||
| Stasis | 1-log kill | Stasis | 1-log kill | Stasis | 1-log kill | Stasis | 1-log kill | ||
| S. aureus | Low | 11.4 ± 0.8 | 15.3 ± 1.0 | 16.9 ± 3.4 (169) | 71.6 ± 14.4 (716) | 25.1 ± 5.1 (32.2) | 101 ± 21 (129) | 24.5 ± 4.0 (33.1) | 62.6 ± 10.1 (84.6) |
| High | 16.1 ± 0.9 | 19.8 ± 1.1 | 60.8 ± 11.2 (608) | 99.2 ± 18.3 (992) | 109 ± 20 (140) | 249 ± 46 (319) | 157 ± 23 (212) | 295 ± 44 (399) | |
| S. pneumoniae | Low | 8.39 ± 1.87 | 13.8 ± 3.1 | 4.62 ± 0.67 (46.2) | 8.51 ± 1.22 (85.1) | 30.1 ± 2.8 (38.6) | 60.7 ± 5.6 (77.8) | 12.6 ± 2.6 (17.0) | 28.2 ± 5.8 (38.1) |
| High | 16.7 ± 1.2 | 18.4 ± 1.3 | 6.66 ± 0.84 (66.6) | 13.4 ± 1.7 (134) | 59.8 ± 5.9 (76.7) | 127 ± 13 (163) | 16.8 ± 3.2 (22.7) | 28.9 ± 5.5 (39.1) | |
Time course of in vivo antibacterial activity of vancomycin.
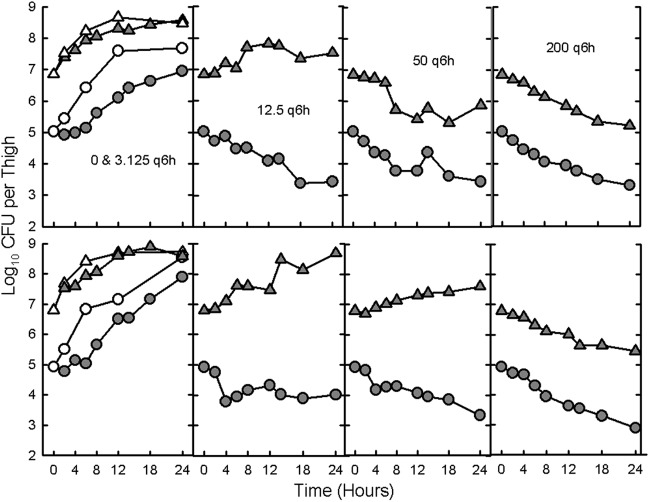
The time courses of dosing every 6 hours of 4 different doses of VAN at LI and HI for 2 strains of MRSA (19936 and 11888) in neutropenic mice are shown in Fig. 2. Killing at HI started at doses that were 4- and 16-fold higher than those resulting in killing at LI. The in vivo killing rates were also lower at HI than at LI (Table 5). In vivo growth over the first 6 h was similar at HI and LI (1.4 log10 for both inocula of MRSA 19936 and 1.9 log10 versus 1.8 log10, respectively, for MRSA 11888). However, growth after 6 h was much lower at HI than at LI (0.3 log10 versus 1.7 log10 for MRSA 19936 and 0.3 log10 versus 1.3 log10 for MRSA 11888). The difference between in vivo growth and killing at most doses of vancomycin was already apparent by 6 h.
Fig 2.
In vivo time course of antibacterial activity of vancomycin at 3.125, 12.5, 50, and 200 mg/kg, administered s.c. every 6 h at both low (solid circles) and high (solid triangles) inocula of MRSA 19931 (Fig. 2A) and MRSA 11888 (Fig. 2B) injected into opposite thighs of neutropenic mice. Open circles and triangles represent the growth in saline-treated controls.
Table 5.
Change in log10 CFU/thigh per hour for in vivo and in vitro growth and killing of MRSA 19936 and 11888 exposed at low and high inocula to vancomycina
| Expt type and strain | Inoculum | Change in log10 CFU/thigh per hour in control or with each dose |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | 3.125 mg/kg q6h | 12.5 mg/kg q6h | 50 mg/kg q6h | 200 mg/kg q6h | 1× the MIC | 2× the MIC | 4× the MIC | 8× the MIC | ||
| In vivo | ||||||||||
| MRSA 19936 | Low | 0.215 | 0.097 | −0.072 | −0.059 | −0.070 | ||||
| High | 0.143 | 0.064 | 0.031 | −0.060 | −0.073 | |||||
| MRSA 11888 | Low | 0.197 | 0.138 | −0.028 | −0.058 | −0.088 | ||||
| High | 0.162 | 0.076 | 0.080 | 0.038 | −0.060 | |||||
| In vitro | ||||||||||
| MRSA 19936 | Low | 0.310 | −0.083 | −0.107 | −0.112 | −0.110 | ||||
| High | 0.166 | −0.071 | −0.080 | −0.082 | −0.081 | |||||
| MRSA 11888 | Low | 0.262 | −0.091 | −0.098 | −0.105 | −0.102 | ||||
| High | 0.112 | −0.052 | −0.071 | −0.073 | −0.073 | |||||
Negative numbers reflect killing. q6h, every 6 h.
In vitro kill curves with vancomycin.
In vitro kill curves for VAN at increasing concentrations against MRSA 11888 in MH broth at HI and LI are shown in Fig. 3. The in vitro growth rates were similar at both inocula over the first 4 h. However, there was no in vitro difference for when killing began between the two inocula. The only effect of HI on in vitro activity of VAN was a lower rate of killing (Table 5). Similar results were obtained with MRSA 19936, as shown in Table 5.
Fig 3.
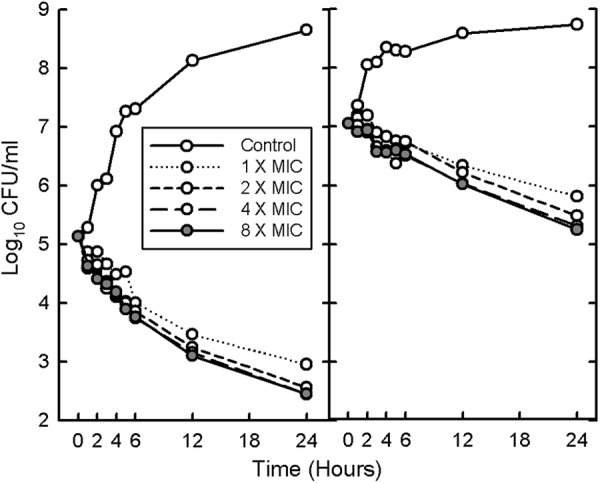
In vitro time course of antibacterial activity of vancomycin at 1, 2, 4, and 8 times the MIC at both low (left) and high (right) inocula of MRSA 11888. MRSA 19936 showed a similar pattern.
DISCUSSION
Vancomycin has been considered to be one of the standard drugs for the treatment of infections during the era of increasing multidrug-resistant Gram-positive organisms (23–25). However, VAN failure rates among patients with endocarditis, bacteremia, or bacteremic pneumonia due to S. aureus have ranged from 37% to as high as 58% (8, 26, 27). Several studies have observed no differences in the MICs of the MSSA and MRSA strains between patients who failed therapy and those who had a successful outcome (28–31). The reasons for the failure are probably multifactorial. Some of the factors previously associated with a poor outcome include low bactericidal activity in in vitro killing assays (32), poor pharmacokinetic properties, such as low penetration into epithelial lining fluid (33), AUC0-24/MIC ratios less than 400 (34), a VAN trough level of <15 mg/liter (5, 27), and an increase or gradual rise in MICs to values of 2.0 mg/liter (27, 35). Our studies suggest that moderately high inocula are another factor that can have a negative effect on the in vivo efficacy of VAN.
Previous studies with Gram-negative bacilli have not observed any significant inoculum effect with inocula from 105 to 108 for several antimicrobials (36, 37). This study also confirms our previous experience that a significant inoculum effect is not observed with Streptococcus pneumoniae within this range of inocula. However, our results with DAP, LZD, and VAN against both MSSA and MRSA strains demonstrate a significant inoculum effect with these organisms. CFB resulted in the smallest inoculum effect with the various strains of S. aureus. Prior reports of the total drug AUC0-24/MIC for VAN to produce stasis in the neutropenic murine thigh model with inocula around 107 have ranged from 86 to 460 (28, 38). Lower values around 25 were observed in this study at an inoculum of 105. Our previously published fAUC0-24/MIC values for DAP and total drug AUC0-24/MIC values for LZD in the same neutropenic murine thigh model against S. aureus at inocula around 106 to 107 for stasis ranged from 39 to 54 (mean = 44) and from 75 to 237 (mean = 89), respectively (20, 22). Slightly higher fAUC0-24/MIC values were observed in these studies for staphylococci with both drugs (mean = 61 for DAP and 106 for LZD), but the mean starting inocula were also 0.3 to 0.5 log10 CFU/thigh higher in the current study. Much lower mean fAUC0-24/MIC values of 17 for DAP and 25 for LZD were observed at the LI. In fact, the fAUC0-24/MIC values at LI for DAP, LZD, and VAN with staphylococci are very close to what one would expect if unbound drug concentrations in serum averaged 1 times the MIC for 24 h. In contrast, at the HI, one would need to average more than 3, 4, and 6 times the MIC to just get stasis with DAP, LZD, and VAN, respectively.
Most reports studying IE have used more than 107 CFU as the HI for simulation of abscesses or vegetations in infective endocarditis (11, 12, 39). LaPlante and Rybak found that very HI (9.5 log10 CFU/g) significantly reduced the killing rates of VAN and nafcillin against MRSA strains in an in vitro pharmacodynamic model with simulated endocarditis vegetations. Vancomycin demonstrated bactericidal activity as early as 32 h after administration for LI (105.5) but did not achieve bactericidal activity throughout the 72 h of study for HI. The inoculum appeared to influence the activity of drugs in the following order: nafcillin > VAN > DAP > LZD (12). It has been known that cell wall active agents have large IEs at very high inocula since most organisms are in the stationary growth phase and one needs growing bacteria to observe bacterial killing (7, 11). We chose to compare inocula of 105 and 107 because we did not want early differences in the growth rate to affect our results. In our in vitro time-kill studies with VAN, the initial growth rates and the onset of killing were similar at both inocula but the rate of killing was lower at 107 CFU/ml than at 105 CFU/ml. However, in vivo, the onset of killing at HI occurred at 4- to 16-fold-higher dosages of VAN than observed at LI. Once killing was started, we still observed a lower in vivo killing rate by VAN at HI than at LI. In these studies, we observed similar growth rates over the first 6 h in saline-treated mice at both LI and HI; growth rates after 6 h were much lower at HI than at LI. However, at several VAN dosages, growth was already observed at 6 h at HI while killing was seen in the opposite thigh at LI. Thus, we do not think our observations can be explained entirely by different in vivo growth rates at LI and HI.
Another possible explanation is the rapid reduction of free VAN concentrations at very HI of staphylococci caused by an excessive binding of the drug to staphylococci and their products (40). The antibacterial target for VAN is the d-alanyl-d-alanine dipeptide of the membrane muropeptides (41). However, there are large amounts of d-ala-d-ala sequences in the preexisting cell wall, and binding to this d-ala-d-ala has no antibacterial effect and the sequence behaves as a false target. VAN binds to both true and false d-ala-d-ala targets. It is calculated that each S. aureus cell has 106 false targets and 103 to 104 true targets (41). At 100-fold-higher inocula, there will be 100-fold-higher density of both the true and false targets but the same number of drug molecules. One would expect that binding to the increased number of false targets may decrease the activity of vancomycin. Extract staphylococcal slime has also been shown to reduce the activity of vancomycin 4-fold (42). Early slime and biofilm formation may also explain the inoculum effect observed with staphylococci and the difference among various strains (43). Previous studies have demonstrated that the ultrastructure of staphylococci in vivo is comparable to that of staphylococci grown on a solid support medium, such as a membrane, and different from that of staphylococci grown in a liquid medium (44). Organisms growing on a surface are usually more resistant to killing by antibiotics (43). This may explain why a larger inoculum effect is observed with most antibiotics with staphylococci in vivo than in vitro when comparing 105 and 107 CFU/thigh or ml.
Another observation from our in vivo studies is the lower bacterial killing of staphylococci at LI than at HI. Staphylococci are known to be taken up by a variety of cells, including endothelial cells, fibrocytes, and monocytes (45, 46). We suspect that the percentage of bacteria taken up by these cells may saturate at lower inocula. Thus, at HI, a higher percentage of the staphylococci would be in extracellular sites than would be at LI. In addition, the killing of intracellular staphylococci by DAP, LZD, and VAN is quite low and varies from 0.4 log10 to 1.0 log10 CFU/cell over 24 h (45, 47).
Our data with inocula around 107 CFU/thigh are close to the pharmacodynamic targets observed with serious infections in patients treated with VAN. For example, stasis and a 1-log kill of S. aureus for VAN required total drug AUC0-24/MIC ratios of 212 and 399, respectively. Two retrospective reviews of MRSA bacteremia with and without associated endocarditis treated with VAN identified AUC0-24/MIC values of 211 and 421 by classification and regression tree (CART) analysis that best differentiated success from failure in their respective patient populations (27, 48). The total drug AUC0-24/MIC values observed in mice for stasis and 1-log kill with LZD were 140 and 319, respectively. The only pharmacodynamic clinical efficacy analysis for bacteremia with LZD reported a median AUC0-24/MIC value of 128 for eradicated cases and 65 failed cases (49). However, most of the patients had infections due to vancomycin-resistant enterococci rather than to MRSA. There are, as yet, no reported clinical studies on a pharmacodynamic target for efficacy of daptomycin therapy.
In conclusion, IE was observed most profoundly with VAN compared to with CFB, DAP, and LZD (P < 0.05) in S. aureus, regardless of oxacillin resistance. The increase in the static dose for VAN ranged from 6.3- to 20.3-fold. The increase in the static dose with all other drugs ranged only from 1.7- to 9.3-fold. The IEs of all 4 drugs for strains of S. pneumoniae were similar and not altered by penicillin resistance. The AUC0-24/MIC values for VAN observed for S. aureus at HI tended to reflect pharmacodynamic targets for efficacy identified in retrospective clinical trials of patients with serious MRSA infections. Studies with newer antistaphylococcal drugs should also include animal efficacy studies at HI. Our data also suggest that patients with LI (around 105) infections, for which the Gram stain shows only a few staphylococci, may be effectively treated with VAN at much lower AUC0-24/MIC targets.
ACKNOWLEDGMENTS
We thank Alastair MacGowan and Karen Bowker, Department of Microbiology, University of Bristol, Bristol, United Kingdom, for providing all the MRSA isolates used in this study.
Footnotes
Published ahead of print 7 January 2013
REFERENCES
- 1. Carratala J, Mykietiuk A, Fernandez-Sabe N, Suarez C, Dorca J, Verdaquer R, Manresa F, Gudiol F. 2007. Health care-associated pneumonia requiring hospital admission: epidemiology, antibiotic therapy, and clinical outcomes. Arch. Intern. Med. 167:1393–1399 [DOI] [PubMed] [Google Scholar]
- 2. Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, Harrison LH, Lynfield R, Dumyati G, Townes JM, Craig AS, Zell ER, Fosheim GE, McDougal LK, Carey RB, Fridkin SK, Active Bacterial Core Surveillance (ABCs) MRSA Investigators 2007. Invasive methicillin-resistant Staphylococcus aureus infection in the United States. JAMA 298:1763–1771 [DOI] [PubMed] [Google Scholar]
- 3. Craig WA. 1998. Pharmacokinetics and pharmacodynamics of antibiotics in mice and men. Clin. Infect. Dis. 26:1–12 [DOI] [PubMed] [Google Scholar]
- 4. Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BM, Rybak MJ, Talan DA, Chambers HF. 2011. Clinical practice guidelines by the Infectious Disease Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. 53:285–292 [DOI] [PubMed] [Google Scholar]
- 5. Rybak MJ, Lomaestro BM, Rotschafer JC, Moellering RC, Craig WA, Billeter M, Dalovisio JR, Levine DP. 2009. Vancomycin therapeutic guidelines: a summary of consensus recommendations from the Infectious Disease Society of America, the American Society of Health-System Pharmacists, and the Society of Infectious Diseases Pharmacists. Clin. Infect. Dis. 49:325–327 [DOI] [PubMed] [Google Scholar]
- 6. Stratton CW. 2006. In vitro susceptibility testing versus in vivo effectiveness. Med. Clin. North Am. 90:1077–1088 [DOI] [PubMed] [Google Scholar]
- 7. Soriano F. 1992. Optimal dosage of beta-lactam antibiotics: time above the MIC and inoculum effect. J. Antimicrob. Chemother. 30:566–569 [DOI] [PubMed] [Google Scholar]
- 8. Stevens DL. 2006. The role of vancomycin in the treatment paradigm. Clin. Infect. Dis. 42(Suppl 1):S51–S57 [DOI] [PubMed] [Google Scholar]
- 9. Tice AD, Hoaglund PA, Shoultz DA. 2003. Risk factors and treatment outcomes in osteomyelitis. J. Antimicrob. Chemother. 51:1261–1268 [DOI] [PubMed] [Google Scholar]
- 10. Woods DD. 1940. The relation of p-aminobenzoic acid to the mechanism of the action of sulphanilamide. Br. J. Exp. Pathol. 21:74–90 [Google Scholar]
- 11. Brook I. 1989. Inoculum effect. Rev. Infect. Dis. 11:361–368 [DOI] [PubMed] [Google Scholar]
- 12. LaPlante KL, Rybak MJ. 2004. Impact of high-inoculum Staphylococcus aureus on the activities of nafcillin, vancomycin, linezolid, and daptomycin, alone and in combination with gentamicin, in an in vitro pharmacodynamic model. Antimicrob. Agents Chemother. 48:4665–4672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mizunaga S, Kamiyama T, Fukuda Y, Takahata M, Mitsuyama J. 2005. Influence of inoculum size of Staphylococcus aureus and Pseudomonas aeruginosa on in vitro activities and in vivo efficacy of fluoroquinolones and carbapenem. J. Antimicrob. Chemother. 56:91–96 [DOI] [PubMed] [Google Scholar]
- 14. Craig WA, Andes DR. 2006. In vivo pharmacodynamics of vancomycin against VISA, heteroresistant VISA (hVISA) and VSSA in the neutropenic murine thigh-infection model, p 16, abstr A-644 Abstr. 46th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC [Google Scholar]
- 15. Walsh TR, Bolmstrom A, Qwarnstrom A, Ho P, Wootton M, Howe RA, MacGowan AP, Diekema D. 2001. Evaluation of current methods for detection of staphylococci with reduced susceptibility to glycopeptides. J. Clin. Microbiol. 39:2439–2444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Clinical and Laboratory Standards Institute 2011. Performance standards for antimicrobial susceptibility testing; 21st informational supplement. M100-S21. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 17. National Research Council, Committee on the Care and Use of Laboratory Animals, Institute of Laboratory Animal Resources, Commission on Life Sciences 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, DC [Google Scholar]
- 18. Andes D, Craig WA. 2002. Animal model pharmacokinetics and pharmacodynamics: a critical review. Int. J. Antimicrob. Agents 19:261–268 [DOI] [PubMed] [Google Scholar]
- 19. Craig WA, Suh B. 1991. Protein binding and the antimicrobial effects: methods for the determination of protein binding, p 367–402 In Lorian V. (ed), Antibiotics in Laboratory Medicine, 3rd ed Lippincott Williams & Wilkins, Baltimore, MD [Google Scholar]
- 20. Andes D, van Ogtrop ML, Jang J, Craig WA. 2002. In vivo pharmacodynamics of a new oxazolidinone (linezolid). Antimicrob. Agents Chemother. 46:3484–3489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Craig WA, Andes DR. 2008. In vivo pharmacodynamics of ceftobiprole against multiple bacterial pathogens in murine thigh- and lung-infection models. Antimicrob. Agents Chemother. 52:3492–3496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Safdar N, Andes D, Craig WA. 2004. In vivo pharmacodynamic activity of daptomycin. Antimicrob. Agents Chemother. 48:63–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cunha BA. 2006. Antimicrobial therapy of multidrug-resistant Streptococcus pneumoniae, vancomycin-resistant enterococci, and methicillin-resistant Staphylococcus aureus. Med. Clin. North Am. 90:1165–1182 [DOI] [PubMed] [Google Scholar]
- 24. Klugman KP, McGee L. 2007. Resurgence of the multiresistant pneumococcus in the United States: a commentary. Pediatr. J. Infect. Dis. 26:473–474 [DOI] [PubMed] [Google Scholar]
- 25. Levine DP. 2006. Vancomycin: a history. Clin. Infect. Dis. 42:S5–S12 [DOI] [PubMed] [Google Scholar]
- 26. Kollef MH. 2007. Limitations of vancomycin in the management of resistant staphylococcal infections. Clin. Infect. Dis. 45:S191–S195 [DOI] [PubMed] [Google Scholar]
- 27. Kullar R, Davis SL, Levine DP, Rybak MJ. 2011. Impact of vancomycin exposure on outcomes in patients with methicillin-resistant Staphylococcus aureus bacteremia: support for consensus guidelines suggested targets. Clin. Infect. Dis. 52:975–981 [DOI] [PubMed] [Google Scholar]
- 28. Craig WA. 2003. Basic pharmacodynamics of antibacterials with clinical applications to the use of β-lactams, glycopeptides, and linezolid. Infect. Dis. Clin. North Am. 17:479–501 [DOI] [PubMed] [Google Scholar]
- 29. Hawkins C, Huang J, Jin N, Noskin GA, Zembower TR, Bolon M. 2007. Persistent Staphylococcal aureus bacteremia: an analysis of risk factors and outcomes. Arch. Intern. Med. 167:1861–1867 [DOI] [PubMed] [Google Scholar]
- 30. Wang J-T, Wang J-L, Fang B-T, Chie W-C, Lai M-S, Lauderdale T-L, Weng C-M, Chang S-C. 2010. Risk factors for mortality of nosocomial methicillin-resistant Staphylococcus aureus (MRSA) bloodstream infection: with investigation of the potential role of community-associated MRSA strains. J. Infect. 61:449–457 [DOI] [PubMed] [Google Scholar]
- 31. Wysocki M, Delatour F, Faurissson F, Rauss A, Pean Y, Misset B, Thomas F, Timsit JF, Similowski T, Mentec H, Mier L, Dreyfuss D. 2001. Continuous versus intermittent infusion of vancomycin in severe staphylococcal infections: prospective multicenter randomized study. Antimicrob. Agents Chemother. 45:2460–2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sakoulas G, Moise-Broder PA, Schentag J, Forrest A, Moellering RC, Jr, Eliopoulos GM. 2004. Relationship of MIC and bactericidal activity to efficacy of vancomycin for treatment of methicillin-resistant Staphylococcus aureus bacteremia. J. Clin. Microbiol. 42:2398–2402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lamer C, De Beco V, Soler P, Calvat S, Fagon J-Y, Dombret M-C, Farinotti R, Chastre J, Gilbert C. 1993. Analysis of vancomycin entry into pulmonary lining fluid by bronchoalveolar lavage in critically ill patients. Antimicrob. Agents Chemother. 37:281–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Moise-Broder PA, Forrest A, Birmingham MC, Schentag JJ. 2004. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin. Pharmacokinet. 43:925–942 [DOI] [PubMed] [Google Scholar]
- 35. Sader HS, Fey PD, Limaye AP, Madinger N, Pankay G, Rahal J, Rybak MJ, Snydman DR, Steed LL, Waites K, Jones RN. 2009. Evaluation of vancomycin and daptomycin potency trends (MIC creep) against methicillin-resistant Staphylococcus aureus isolates collected in nine U.S. medical centers from 2002 to 2006. Antimicrob. Agents Chemother. 53:4127–4132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Craig WA, Bhavnani SM, Ambrose PG. 2004. The inoculum effect: fact or artifact? Diagn. Microbiol. Infect. Dis. 50:229–230 [DOI] [PubMed] [Google Scholar]
- 37. Maglio D, Ong C, Barevicius GQ, Nightingale CH, Nicolau DP. 2004. Determination of the in vivo profile of cefepime against extended-spectrum-β-lactamase-producing Escherichia coli at various inocula. Antimicrob. Agents Chemother. 48:1941–1947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rybak MJ. 2006. The pharmacokinetic and pharmacodynamic properties of vancomycin. Clin. Infect. Dis. 42(Suppl 1):S35–S39 [DOI] [PubMed] [Google Scholar]
- 39. Stevens DL, Yan S, Bryant AE. 1993. Penicillin-binding protein expression at different growth stages determines penicillin efficacy in vitro and in vivo: an explanation for the inoculum effect. J. Infect. Dis. 167:1401–1405 [DOI] [PubMed] [Google Scholar]
- 40. Ekdahl C, Hanberger H, Hallgren A, Nilsson M, Svensson E, Nilsson LE. 2005. Rapid decrease of free vancomycin in dense staphylococcal cultures. Eur. J. Clin. Microbiol. Infect. Dis. 24:596–602 [DOI] [PubMed] [Google Scholar]
- 41. Hiramatsu K, Hanuki H. 1998. Glycopeptide resistance in staphylococci. Curr. Opin. Infect. Dis. 11:653–658 [DOI] [PubMed] [Google Scholar]
- 42. Mathur T, Singhal S, Khan S, Upadhyay D, Fatma T, Rattan A. 2005. Adverse effect of staphylococcal slime on in vitro activity of glycopeptide. Jpn. J. Infect. Dis. 58:353–357 [PubMed] [Google Scholar]
- 43. Schwank S, Rajacic Z, Zimmerli W, Blaser J. 1998. Impact of bacterial biofilm formation on in vitro and in vivo activities of antibiotics. Antimicrob. Agents Chemother. 42:895–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lorian V, Zak O, Sutter J, Bruecher C. 1985. Staphylococci in vitro and in vivo. Diagn. Microbiol. Infect. Dis. 3:433–444 [DOI] [PubMed] [Google Scholar]
- 45. Lemaire S, van Bambeke F, Pievard D, Applebaum PC, Tulkens PM. 2011. Activity of fusidic acid against extracellular and intracellular Staphylococcus aureus: influence of pH and comparison with linezolid and clindamycin. Clin. Infect. Dis. 52(Suppl 7):S493–S503 [DOI] [PubMed] [Google Scholar]
- 46. Vesga O, Groeschel MC, Otten MF, Brar DW, Vann JM, Proctor RA. 1996. Staphylococcus aureus small colony variants are induced by the endothelial cell intracellular milieu. J. Infect. Dis. 173:739–742 [DOI] [PubMed] [Google Scholar]
- 47. Brinch KS, Tulkens PM, van Bambeke F, Frimodt-Moller N, Hoiby N, Kristensen HH. 2010. Intracellular activity of the peptide antibiotic NZ 2114: studies with Staphylococcus aureus and human THP-1 monocytes and comparison with daptomycin and vancomycin. J. Antimicrob. Chemother. 65:1720–1724 [DOI] [PubMed] [Google Scholar]
- 48. Brown J, Brown K, Forrest A. 2012. Vancomycin AUC0-24/MIC ratio in patients with complicated bacteremia and infective endocarditis due to methicillin-resistant Staphylococcus aureus and its association with attributable mortality during hospitalization. Antimicrob. Agents Chemother. 56:634–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rayner CR, Forrest A, Meagher AK, Birmingham MC, Schentag JJ. 2003. Clinical pharmacodynamics of linezolid in seriously ill patients treated in a compassionate use program. Clin. Pharmacokinet. 42:1411–1423 [DOI] [PubMed] [Google Scholar]