Fig 6.
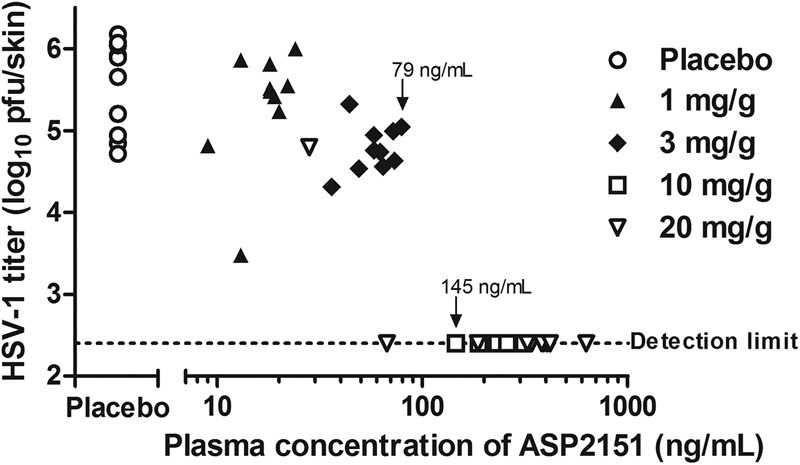
Relationship between intradermal HSV-1 titer and ASP2151 concentration in plasma in mice continuously infused with ASP2151. HSV-1-infected mice (10 animals per group) were infused for 5 days with placebo (vehicle) or ASP2151 solution (1, 3, 10, or 20 mg/g dissolved in PEG 400) starting 1 to 2 h before HSV-1 infection. Intradermal HSV-1 titer (log10 PFU/skin) was determined by plaque assay at day 5 postinfection and was then plotted for each animal. The dotted line indicates the lower limit of detection (2.40 log10 PFU/skin) for the assay. The highest maintained concentration in plasma at which the intradermal HSV-1 titer could be detected and the lowest maintained concentration in plasma to reduce the intradermal HSV-1 titer below the detection limit are indicated (79 and 145 ng/ml, respectively).
