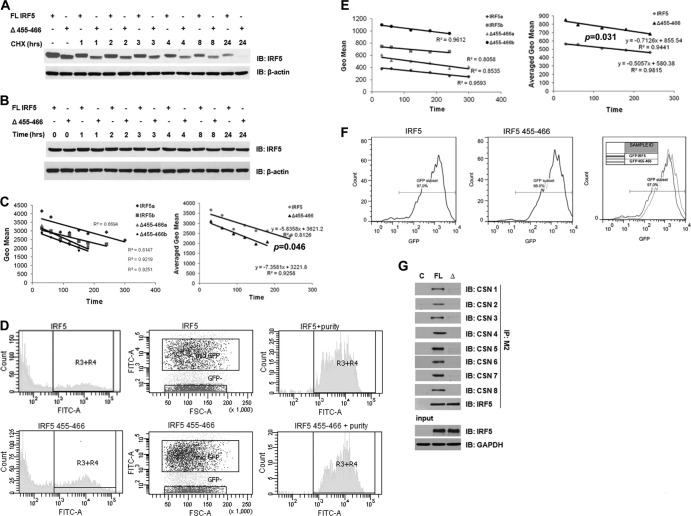
Fig 4.
The CSN/IRF5 interaction controls IRF5 protein stability. (A) Plasmids encoding full-length (FL) and mutant (Δ455-466) Flag- or GFP-tagged IRF5 were transiently transfected to HEK293T cells and chased with CHX. Cells were harvested at the indicated time points after CHX treatment, and proteins were separated by SDS-PAGE. Protein levels were determined by immunoblot analysis with anti-IRF5 and anti-β-actin antibodies. (B) Same as panel A, except that cells were left untreated and harvested at each indicated time point. (C) Cells expressing medium levels of GFP-IRF5 were sorted and treated with CHX for the indicated time periods and then gated on by flow cytometry to determine intracellular levels of IRF5. GFP intensity was quantified at each time point and plotted on a graph as a function of time. The rate of exogenous protein decay was determined by fitting a line to each data set and calculating the slope. Representative results from two independent replicates are shown (IRF5a and b, Δ455-466a and b); the average rate of decay was calculated from three independent experiments performed in duplicate. P was <0.05 by paired two-tailed Student's t test. (D) Representative histograms of fluorescence distribution and sorting of GFP-tagged full-length and mutant IRF5 proteins expressing medium levels of GFP (R3+R4). The purity of sorted cells that were treated with CHX is shown. FSC, forward scatter. (E) Same as in panel C, except that total cells transfected with either plasmid were treated with CHX over the indicated time periods and IRF5 levels were determined by flow cytometry. Geo mean, geometric mean. (F) Same as in panel D, except that representative histograms show the strategy of gating on GFP-IRF5-positive cells. The geometric mean was obtained from the subset of cells indicated in the fluorescence histograms. An overlay of the two experimental conditions is shown. (G) Full-length (FL) and mutant (Δ) GFP-IRF5 were transiently transfected to HEK293T cells, lysates were immunoprecipitated with anti-IRF5 antibodies, and interaction with individual COP9 subunits was determined by immunoblotting. C, control.