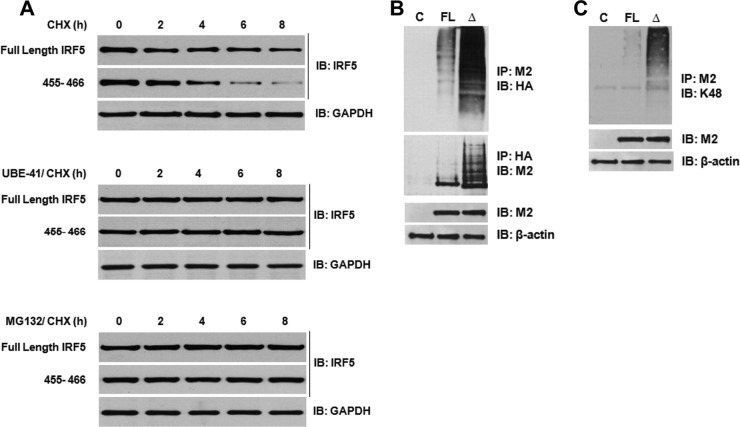
Fig 6.
IRF5 degradation is abrogated by inhibition of ubiquitin-activating enzyme 1 (E1) or the proteasome. (A) Plasmids encoding full-length IRF5 or the Δ455-466 mutant were transiently transfected to HEK293T cells and treated with CHX or CHX plus UBEI-41 (ubiquitin E1 inhibitor) or MG132 (proteasome inhibitor). The rate of IRF5 and GAPDH protein decay was examined by immunoblot analysis. The top set of panels represents CHX-treated cells, and the bottom two sets of panels are combined treatments with each inhibitor. (B) Similar to panel A, except that the HA-Ub plasmid was cotransfected with plasmids encoding an empty vector (control [C]), full-length IRF5 (FL), or Δ455-466 mutant IRF5 (Δ) to HEK293T cells and treated with MG132 to examine IRF5 ubiquitination. Reciprocal immunoprecipitations are shown by pulling down IRF5 (M2) or ubiquitin (HA) and examining the alternate. Levels of IRF5 and β-actin in whole-cell lysates used for immunoprecipitations are shown. (C) Similar to panel B, except that cells were not transfected with HA-Ub and levels of IRF5 K48-linked ubiquitination were determined by immunoblotting M2 precipitates with anti-K48-linked polyubiquitination antibodies. Expression of IRF5 and β-actin in whole-cell lysates used for the immunoprecipitations is shown.