Fig 4.
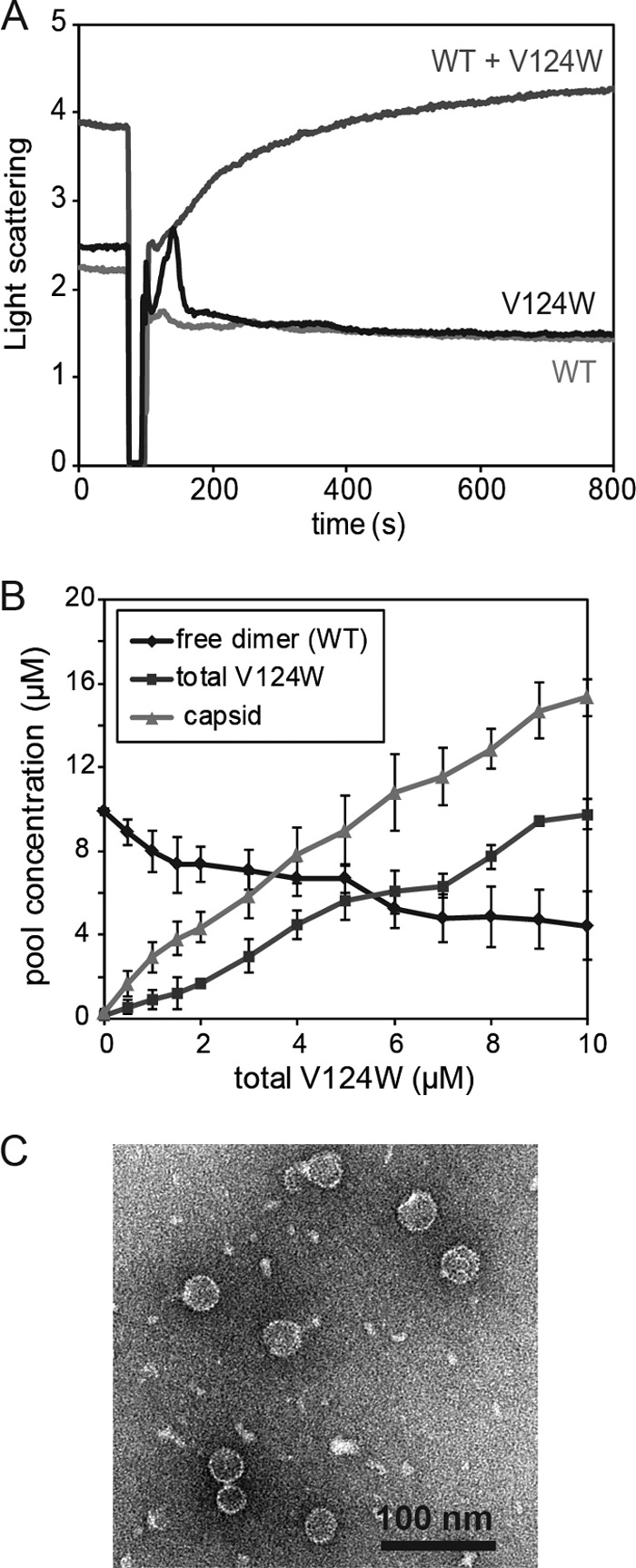
WT and V124W dimers coassemble into capsids in vitro. (A) Coassembly kinetics of 10 μM WT and 1 μM V124W mutant at 100 mM NaCl and 23°C was monitored by 90° light scattering. The proteins alone (10 μM WT or 1 μM V124W mutant) did not assemble under these conditions. However, if the two proteins were mixed together, time-dependent coassembly kinetics was observed. Each light-scattering trace shows the average for four independent experimental results. (B) Coassembly thermodynamics at 100 mM NaCl and 23°C. In the absence of the V124W mutant, 10 μM WT dimers did not assemble. With increasing concentrations of the V124W mutant, the free WT dimer concentration decreased and the yield of capsids increased. The total capsid concentration exceeded the total concentration of the V124W mutant, indicating coassembly. Each point shows the average for three to five independent experimental results. (C) Negatively stained electron micrographs of 10 μM WT and 5 μM V124W mutant coassembly products. No aberrant structures were observed.
