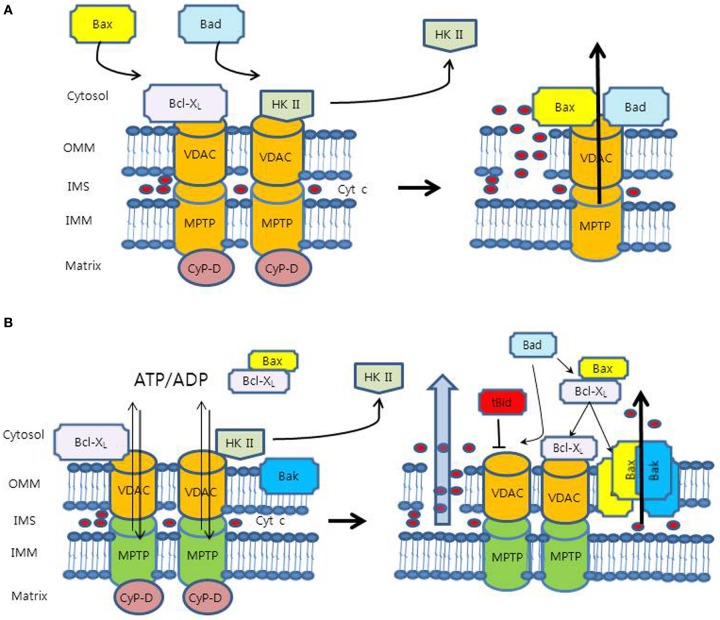
Figure 1.
Proposed models for mechanism of the mitochondrial outer membrane permeabilization (MOMP). (A) VDAC is the OMM component of the MPTP complex. In this model, pro-apoptotic proteins (Bax and Bad) interact with VDAC to accelerate its opening, whereas Bcl-XL binds to VDAC directly to close it. VDAC can cause cyt c release indirectly through the swelling and rupture of the OMM. However, this model has problems of relatively small pore size and dispensability of VDAC. (B) In an anti-apoptotic state, anti-apoptotic molecules (HK II and Bcl-XL) bind to VDAC and keep it in open configuration with low-conductance for the exchange of adenine nucleotides, which maintains OMM integrity. HK II competes with Bcl-XL for binding site of VDAC. In a pro-apoptotic state, HK II detachment from VDAC promotes binding of Bcl-XL to VDAC, releasing Bax from Bcl-XL. Free Bax interacts with Bax/Bak to form pore structures for the release of cyt c. Bad interacts with Bcl-XL on Bcl-XL/Bax and Bcl-XL/VDAC, which release Bax from Bcl-XL/Bax heterodimer to form Bax/Bak and displace Bcl-XL from VDAC to be sensitized to Ca2+, respectively. In this model, tBid was shown to induce VDAC closure, reducing adenine nucleotide exchange and creating mitochondrial dysfunction, which may cause the MOMP.