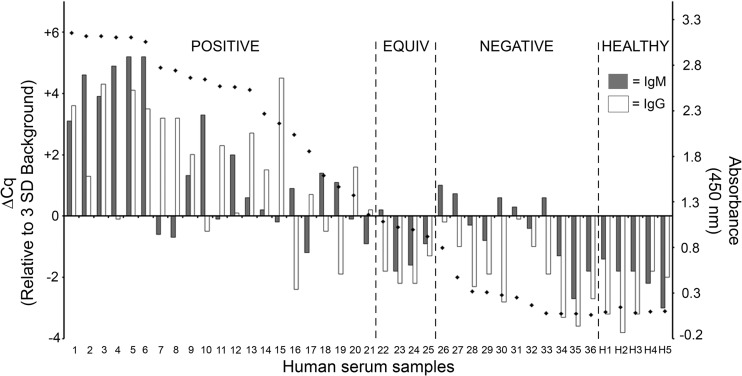
Fig 5.
Recombinant antigen iPCR successfully quantified B. burgdorferi VlsE C6 peptide antibodies in human serum samples. Results are for 36 serum samples from 18 Lyme disease patients collected upon the initial visit to a clinic and at a 10-day follow-up visit and 5 healthy controls using a multiplex iPCR protocol to quantitate both IgM and IgG isotypes using recombinant B. burgdorferi VlsE C6 peptide-coated magnetic beads. A call threshold (ΔCq = 0) was assigned at greater than or equal to 3 standard deviations above the mean background signal determined using sera from healthy individuals. Serum samples were also tested using a commercial C6 ELISA (Immunetics, Boston, MA) (diamonds), which was performed according to the manufacturer's protocol, with a call threshold for an absorbance (450 nm) of 1.1 used according to the manufacturer's protocol. The C6 ELISA value represents combined measurement of C6 IgM and IgG antibodies. The data for patients 1 to 36 are grouped into three categories: positive, equivocal (equiv), and negative according to the C6 ELISA values. Samples H1 to H5 correspond to the sera collected from the healthy controls and are grouped accordingly (healthy). The calculated rs values were 0.734 (P < 0.0001) for C6 iPCR IgM versus C6 ELISA, 0.826 (P < 0.0001) for C6 iPCR IgG versus C6 ELISA, and 0.895 (P < 0.0001) for C6 iPCR IgM and/or IgG versus C6 ELISA.