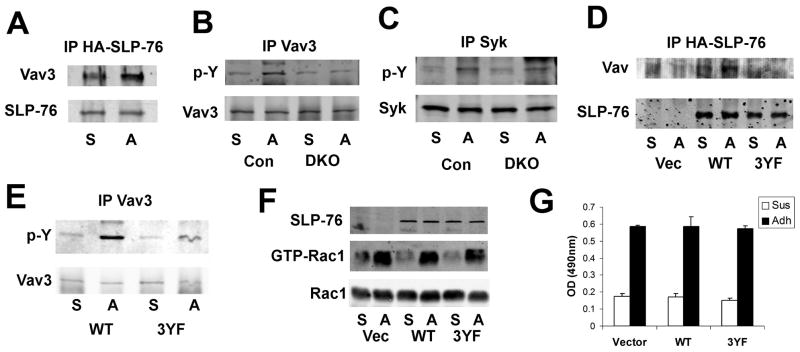
Figure 6. SLP-76 links Syk to Vav3 following integrin engagement in the OC.
BMMs were differentiated into pre-OCs by 3 days culture with M-CSF and RANKL. They were serum and cytokine starved, lifted, and either maintained in suspension (S) or adhered to vitronectin (A). (A) HA-immunoprecipitates of DKO pre-OCs transduced with HA-tagged SLP-76, immunoblotted for Vav and SLP-76, as loading control. (B–C) Lysates of DKO and control (SLP-76+/?BLNK−/−) pre-OCs were immunoprecipitated with an anti-Vav3 (B) or anti-Syk (C) antibody. The immunoprecipitates were immunoblotted for phosphotyrosine and Vav3 (B) or Syk (C) as loading controls. (D–G) DKO BMMs were transduced with WT or 3YF-SLP-76 constructs or empty vector prior to three-day culture with M-CSF and RANKL. (D) Lysates were immunoprecipitated with an anti-HA antibody. Immunoprecipitates were immunoblotted for Vav and SLP-76. (E) Lysates of transduced DKO pre-OCs were immunoprecipitated with an anti-Vav3 Immunoprecipitates were immunoblotted for phosphotyrosine and Vav3. (F–G) Lysates of transduced DKO pre-OCs were (F) subjected to GST-PAK pull-down, followed by Rac1 immunoblot or (G) used to quantitate GTP-Rac1/2 by ELISA. In (F), Rac1 serves as loading control.