Abstract
A survey was conducted to determine the assemblage and abundance of plant-parasitic nematodes and their associations with soil factors in organically farmed fields in Minnesota. A total of 31 soil samples were collected from southeast (SE), 26 samples from southwest (SW), 28 from west-central (WC), and 23 from northwest (NW) Minnesota. The assemblage and abundance of plant-parasitic nematodes varied among the four regions. The soybean cyst nematode, Heterodera glycines, the most destructive pathogen of soybean, was detected in 45.2, 88.5, 10.7, and 0% of organically farmed fields with relative prominence (RP) values of 10.3, 26.5, 0.6, and 0 in the SE, SW, WC, and NW regions, respectively. Across the four regions, other common genera of plant-parasitic nematodes were Helicotylenchus (42.6, RP value, same below), Pratylenchus (26.9), Tylenchorhynchus and related genera (9.4), Xiphinema (5.6), and Paratylenchus (5.3). Aphelenchoides, Meloidogyne, Hoplolaimus, Mesocriconema, and Trichodorus were also detected at low frequencies and/or low population densities. The similarity index of plant-parasitic nematodes between two regions ranged from 0.44 to 0.71 and the similarity increased with decreasing distance between regions. The densities of most plant-parasitic nematodes did not correlate with measured soil factors (organic matter, pH, texture). However, the densities of Pratylenchus correlated negatively with % sand, and Xiphinema was correlated negatively with soil pH.
Keywords: Aphelenchoides, Helicotylenchus, Heterodera glycines, Hoplolaimus, Meloidogyne, Mesocriconema, nematode community, organic-farming, Paratylenchus, Pratylenchus, soybean cyst nematode, Trichodorus, Tylenchorhynchus, Xiphinema
Organic production has been increasing steadily in recent years and will continue to grow in Minnesota. In 2010, the Minnesota Department of Agriculture has estimated the number of organic farms in Minnesota at 653 and in 2008 it was eleventh in the nation in organic production (Moyniham, 2010). Organic-cropping systems may have high pest pressures because there are fewer management options as compared with conventional production systems. Plant-parasitic nematodes are one of the important groups of pests on many organic crops. For example soybean cyst nematode (SCN), Heterodera glycines, causes greater crop loss than any other soybean (Glycines max) disease (Monson and Schmitt, 2004; Wrather and Koenning, 2006). In spite of the widespread availability of resistant cultivars of conventional soybean, management of H. glycines has proven to be difficult, and the distribution and severity of H. glycines continues to increase in the North Central region. Heterodera glycines was first detected in Minnesota in 1979 (MacDonald et al., 1980), and now occurs in most (approximately 65%) conventional soybean fields in southern and central Minnesota, and recently has been detected in a few counties in the Red River Valley in northwest Minnesota (Chen, 2011). Another group of nematodes, lesion nematodes (Pratylenchus spp.), are widely distributed in the United States, have wide host ranges (Mai et al., 1977; Taylor et al., 1958; Taylor and Schleder, 1959), and are also important in conventional fields in Minnesota. Lesion nematodes are migratory endoparasites and involved in disease complexes such as early dying of potatoes (Solanum tuberosum) (Powelson and Rowe, 1993; Rowe and Powelson, 2002), exceeding the disease threshold from one nematode alone.
While management of plant-parasitic nematodes in conventional fields especially for the soybean cyst nematodes using resistant cultivars is widely practiced, little attention has been paid to nematode problems in the organically farmed fields, as most of the resistant soybean cultivars also carry a genetically modified gene for herbicide tolerance and these cultivars cannot be used in organic production systems. Management of insect, diseases, and nematodes in organic-farming systems may need to rely on the inherent equilibrium in nature (Delate, 2003). To achieve the natural low equilibrium level of pests and pathogens, farming practices must include diverse rotations, cover crops, organic matter maintenance/additions, and other practices to promote diverse microbial organisms for a healthy soil. Unlike conventional systems, continuous cropping or a simple corn(Zea mays)-soybean rotation that is commonly practiced in the Midwest in the USA in conventional fields is not allowed in the organic-farming system by the National Organic Program, the federal agency that governs the organic certification process (USDA, 2012). The multi-year crop rotation if appropriately designed may help manage plant-parasitic nematodes (Bhan et al., 2010). Organically farmed fields with application of organic fertilizer such as animal manure may enhance soil health and natural biocontrol of pests (Birkhofer et al., 2008). Transition from conventional to organic farming can increase populations of beneficial bacterivore nematodes while simultaneously reducing the population of the predominant plant-parasitic nematode, Pratylenchus crenatus (Briar et al., 2007).
Little is known about the extent of the H. glycines and other plant-parasitic nematode problems in the organic-farming system in the Midwest of the USA. The objectives of this study were to assess diversity and abundance of plant-parasitic nematodes in the organically farmed fields in Minnesota, and their associated factors including soil characters, crops, and geographical locations. The main aim of the study was to provide baseline information for determining if management actions are needed in the organically farmed fields, and if the management needs should differ in different regions and fields. We hypothesize that the community of plant-parasitic nematodes in the organically farmed fields differ in different regions and fields within the state of Minnesota because there are significant variations of climates, soil conditions and crops across the state.
Materials and Methods
Sampling: A survey was conducted in the fields on 58 certified organic farms during the crop-growing season in 2006 in southeast, southwest, west-central, and northwest regions in Minnesota (Table 1). Farms were arbitrarily selected from the “Directory of Minnesota Organic Farms 2006” and sampled with consent of the farm owners. On each farm, one or two (from two fields with different cropping sequences) soil samples were collected, for a total of 108 samples (Fig. 1). Each soil sample was consisted of approximately 30 soil cores collected with a 2.5-cm-diameter soil probe to a depth of approximately 20 cm across an area of 0.5 to 2 hectares in a field. Most of the samples were collected during mid-season in August to September, and a few samples were collected at the end of season in October, 2006. After collection, soil samples were shipped within 1-2 days to the laboratory. The soils were stored in a cool room (∼10 °C) and processed within 1-2 days after receiving. Crop sequences were recorded for most of the sampled fields.
Table 1.
Climate, soil characters, and current crops of the sampled organically farmed fields in the four regions in Minnesota.

Fig. 1.
Map of the four regions sampled. The values indicate number of samples taken from each county.
Sample processing: The soil samples were thoroughly mixed. A subsample of 100 cm3 soil was used to extract vermiform nematodes using the hand-decanting and sucrose centrifugal-flotation technique (Jenkins, 1964). Half of the nematodes in a sample were killed in water at 63 °C and fixed in 3.7% formaldehyde (Hooper, 1986), and the other half of a sample was placed in antibiotic solution (100 ppm streptomycin, 30 ppm chlortetracycline, and 20 ppm quinolinol). The samples were stored at 4 °C in a refrigerator. The nematodes in the antibiotic solution were generally alive and easier to identify and count, so they were used in counting. However, when needed, the nematodes in the formaldehyde were used to verify the identification and counting. An aliquot of the sample containing at least 100 nematodes, or all of the nematodes in the antibiotic solution if there were fewer than 100 nematodes, were used for counting nematodes except for the second-stage juveniles (J2) of cyst nematodes, for which all samples in the antibiotic solution were examined. Plant-parasitic nematodes were identified to genera. Cyst nematode J2 morphological characters were briefly examined based on body length and stylet length, and if they fit to H. glycines characters, the nematodes were considered H. glycines because other cyst nematodes are uncommon in Minnesota. A few genera of plant-parasitic nematodes occurring in low abundance were not identified due to insignificant contribution to the communities. The nematodes in the genera Aphelenchus and the family Tylenchidae were considered fungal-feeding nematodes although some of them may also feed on roots, moss, and algae (Siddiqi., 2000; Sohlenius et al., 1977; Yeates et al., 1993). Although the nematodes in Aphelenchoides were considered as fungal-feeding nematodes (Yeates et al., 1993), they were included in plant-parasitic nematodes in this survey because some of them are important pathogens on some crops and ornamental plants in Minnesota (Taylor et al., 1958; Taylor and Schleder, 1959).
For soil samples from southeast, southwest, and west-central regions where H. glycines has been reported, the population densities of eggs from the nematode cysts were also determined. Cysts were initially extracted from a subsample of 100 cm3 soil with hand-decanting and wet-sieving. The cysts with some soil particles and debris caught on 250-μm-aperture sieve were collected, suspended in 63% (w/v) sucrose solution in a 50-ml tube, and centrifuged at 1,100g for 5 minutes. The cysts in the supernatant were collected and crushed in a 40-ml glass tissue grinder. The eggs on the 25-μm-aperture sieve were collected into a 50-ml tube and counted. The samples, in which only eggs or J2 were detected, were re-run for eggs and J2 counts to exclude the possibility of contamination in the first run and confirm the infestation of the field by the nematode.
To confirm that the cyst nematodes were H. glycines, soil samples were bioassayed by planting H. glycines-susceptible soybean in 100 cm3 soil in a cone-tainer, and the presence of cyst nematode females was determined after 1 month. Heterodera trifolii was encountered at a low frequency in crop fields in Minnesota in a previous survey (Taylor et al., 1958), and it is possible that the nematode was present in some soils but could not be detected by briefly observing the J2 due to the presence of H. glycines. Because this nematode is important on some leguminous crops such as white clover, bioassay was also performed to detect H. trifolii. A subsample of up to 10 cm3 soil was taken from each of the cyst-positive samples except for a few samples that had no soil left, and the subsamples were bulked, placed in a 16-cm-diameter pot, and planted to white clover (Trifolium repens), which is a good host of H. trifolii (Holtzmann and Aragaki, 1963) but not of H. glycines (Riggs and Hamblen, 1962; Riggs and Hamblen, 1966). Presence of cyst nematode females on the roots was monitored up to three months after planting.
Soil pH, % organic matter, % sand, % silt, and % clay particles were analyzed using standard laboratory procedures in the Agvise Laboratory, Benson, MN.
Data analysis: Absolute and relative frequencies of occurrence, absolute and relative population densities, and absolute and relative prominence values (Norton., 1978) of individual genera of the plant-parasitic nematodes were calculated for the four regions separately and together. The communities of plant-parasitic nematodes in the four regions were compared with the Bray and Curtis similarity index (Bray and Curtis, 1957):
 |
where S is the similarity index ranging from 0 (no similarity) to 1.0 (complete similarity); A is the sum of population densities of each genus of plant-parasitic nematodes in one region; B is the sum of population densities of each genus of plant-parasitic nematodes in another region; and W is the sum of lower population density of common genera in both regions. Frequency distribution of H. glycines egg population densities (eggs/100 cm3 soil) was determined for the four regions. Correlation analyses were performed to determine the association of the population densities of major plant-parasitic genera with soil factors including pH, % organic matter, % sand, % silt, and % clay. For the correlation analyses, the samples containing no nematodes of a genus were excluded.
Results
Soybean cyst nematode: No cyst nematode females were observed on the clover roots in the bioassay, thus H. glycines was the only cyst nematode detected. Heterodera glycines eggs and/or J2 were detected in 45.2, 88.5, 10.7, and 0% fields in southeast, southwest, west-central, and northwest regions, respectively. The infestation of H. glycines in the soil samples was confirmed with greenhouse bioassay. The frequency distributions of soil samples among classes of egg population densities are summarized in Table 2. In the southeast, 29% of the fields had more than 200 eggs/100 cm3 soil, which can cause yield loss to SCN-susceptible soybean cultivars. In the southwest, 65% of the fields had egg population densities exceeding 200 eggs/100 cm3 soil. In some fields (6% in southeast and 23% in southwest) egg population densities were high (> 5,000 eggs/100 cm3 soil), and could cause significant damage even to SCN-resistant soybean cultivars (Chen et al., 2001a). The egg population densities in the egg-positive fields across the regions with current soybean in 2006 ranged from 25 to 13,625 eggs/100 cm3 soil with an average of 3,023 eggs/100 cm3 soil. The population densities decreased with increasing years of non-host crops (Table 3).
Table 2.
Frequency distribution of soil samples from Minnesota organically farmed fields among classes of Heterodera glycines egg numbers per 100 cm3 of soil in 2006.

Table 3.
Population densities (eggs/100 cm3 soil) of Heterodera glycines in fields with different number of years of non-host crops.

Composition and distribution of major plant-parasitic nematode genera: A total of eleven genera of plant-parasitic nematodes were identified. The nematode genera and their prominence, which indicates the frequency of occurrence and population densities, differed among the four regions (Table 4). In the southeast, common genera included Pratylenchus (41.3, RP value, same below), Helicotylenchus (39.5), Heterodera (10.3), Xiphinema (4.8), and Paratylenchus (3.2); Hoplolaimus, Mesocriconema, and Tylenchorhynchus were observed in some samples with low population densities. In the southwest, common genera were Helicotylenchus (38.5), Heterodera (26.4), Pratylenchus (18.2), Xiphinema (12.0), and Tylenchorhynchus and related genera (4.5); Hoplolaimus, Paratylenchus and Aphelenchoides were detected with low prominence values. In the west central, Helicotylenchus (45.8), Pratylenchus (29.4), Tylenchorhynchus and related genera (10.6), Paratylenchus (6.4), and Meloidogyne (4.8 RP in 2 fields) were common; Heterodera, Hoplolaimus, Aphelenchoides, and Trichodorus were the genera with low prominence values. In the northwest, the most common nematodes were Tylenchorhynchus and related genera (44.7), followed by Helicotylenchus (33.1), Paratylenchus (15.9), and Pratylenchus (4.6); Hoplolaimus, Xiphinema, and Trichodorus were infrequently observed. Across the four regions, the common genera of plant-parasitic nematodes based on relative prominence values were Helicotylenchus (42.6), Pratylenchus (26.9), Tylenchorhynchus and related genera (9.4), Heterodera (9.2), Xiphinema (5.6), and Paratylenchus (5.3).
Table 4.
Distributions and prominence of major plant-parasitic nematodes in organic farms in Minnesota. a

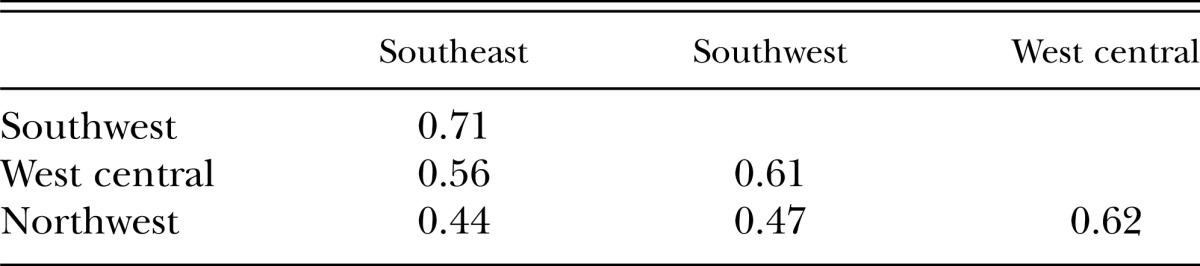
Difference in the communities of plant-parasitic nematodes among the four regions increased with increasing distance between two regions (Table 5). The highest similarity was observed between the two adjacent southern regions (similarity index 0.71) with similar latitude (i.e., similar climatic conditions), and the lowest (similarity index 0.44) was between the southeast and northwest regions, which are farthest apart.
Table 5.
Bray and Curtis similarity index of the plant-parasitic nematodes among the four regions in Minnesota.
Association of the nematode community with edaphic factors: Correlation coefficients of population densities with soil pH, % organic matter, % sand, % silt, and % clay particles are summarized in Table 6. Pratylenchus was positively correlated with % silt (range 6-71%) and negatively correlated with % sand (range 15-78%). Within the range of pH 5.7-7.9, Xiphinema was more abundant in soil with a lower pH than in soil with a higher pH. No significant correlations of other plant-parasitic nematodes with the soil factors were observed.
Table 6.
Correlation coefficients between nematode populations and edaphic factors in the organically farmed fields in Minnesota.

Discussion
In the present survey, a number of plant-parasitic nematodes were detected in the organically farmed fields in Minnesota. The common genera of plant-parasitic nematodes observed in this study were similar to those reported in previous surveys in crop fields in Minnesota (Crow and MacDonald, 1978; MacDonald, 1979; Taylor et al., 1958; Taylor and Schleder, 1959).
Heterodera glycines was first detected in 1979 in Minnesota (MacDonald et al., 1980). This study revealed that H. glycines frequently occurs in the organically farmed fields of certified organic farms in southern Minnesota, especially in southwest region where the percentage of organically farmed fields infested with the nematode is similar to that in conventional soybean fields (S. Chen, 2008, unpublished). While high population densities (>10,000 eggs/100 cm3 soil) occurred in some fields in southern Minnesota, a number of H. glycines-infested soybean fields (64% in southern and 39% in southwest) had egg population densities lower than 1,000 eggs/100 cm3 soil. Previous studies demonstrated that one season of SCN-susceptible soybean can result in a high population density (Chen et al., 2001b) even with a low initial population density. Since no SCN-resistant cultivars were used, the low population densities of H. glycines in the soybean fields indicate the nematode was probably recently introduced, and the population has not yet widely established in the fields. Long rotation with non-host crops may have maintained the H. glycines population densities at a low level in some of the fields that were sampled. The infestation of H. glycines in west-central region has a relatively short history (about 15 years in west-central as compared 30 years in southern (Chen, 2011; MacDonald et al., 1980) and percentage of fields infested was also still low in conventional fields (S. Chen, 2008, unpublished). Although no samples from the northwest region were infested with H. glycines, the risk of H. glycines infestation in organically farmed fields is high because this nematode has been detected in conventional fields in a few counties within the region (Chen, 2011). Extensive soil movement in that region due to crop production practices, frequent flooding, and blowing soil particles will promote the spread of H. glycines in the future.
The soybean cyst nematode is a highly virulent pathogen and can cause severe yield loss to the soybean if it is not appropriately managed (Monson and Schmitt., 2004; Wrather and Koenning, 2006). For conventional fields, H. glycines management mainly relies on the use of SCN-resistant cultivars and rotation with non-host crops (Chen, 2011; MacGuidwin et al., 1995; Niblack, 2005; Niblack and Chen, 2004; Young, 1998). In contrast, there is limited availability of SCN-resistant cultivars for the use in organically farmed fields in Minnesota because organic soybean food products generally require better quality of soybean seeds or specialty type of soybean (for instance Natto) than those produced by SCN-resistant soybean cultivars in conventional fields. Organic farms have more crops in the rotations as compared with most conventional fields because Minnesota organic farmers are required by organic certification rules to use a minimum of a three-crop rotation in order to lower the pressures of diseases and pests. Most of the crops in the rotation are non-hosts or poor hosts of H. glycines. Consequently, crop rotation is currently the most practical option for H. glycines management in organically farmed fields. However, an integrated approach may be needed for effective H. glycines management. Research is needed to determine the effectiveness of various cropping sequences, biological control, and cultural practices such as use of animal and green manures towards alleviating the damage from this nematode in organic production systems. Research efforts should also be directed to developing organic-grade quality of SCN-resistant soybean cultivars for organically farmed field use.
Other cyst nematodes including H. trifolii, Punctodera punctata, and Cactodera sp. were encountered at low frequencies in soil samples from Minnesota in previous surveys (Porter and Chen, 2005; Spears, 1956; Taylor et al., 1958), but they were not detected in this study. Heterodera trifolii was not detected by the bioassay in the sampled fields. However, there is a possibility that other cyst nematodes including Cactodera and Punctodera could be present at low frequencies and/or at low population densities, but could not be separated from H. glycines by briefly observing the J2.
About ten species of lesion nematodes have been identified from crop fields in Minnesota in previous surveys (Taylor et al., 1958; Taylor and Schleder, 1959). The nematodes have a wide range of hosts, are moderately to highly pathogenic to some crops such as potato, corn, and soybean (Mai et al., 1977). Therefore, even with the frequent rotation of crops in organic-farming systems, the nematodes may be able to establish their populations to damaging levels. Further studies, however, are needed to determine the yield loss of various crops to lesion nematodes in organic-farming system and develop effective management strategies.
Two species of dagger nematode, Xiphinema americanum and X. chambersi, were identified in Minnesota in previous studies (Crow and MacDonald, 1978; MacDonald, 1979; Taylor and Schleder, 1959; Wallace and MacDonald, 1979). Xiphinema americanum reported in early studies could be a species complex (Robbins, 1993), and the species identities of Xiphinema in Minnesota may need to be further studied. Xiphinema spp. are large nematodes, and they are highly pathogenic to a number of crops and fruit trees. The nematodes not only cause damage to plants directly by parasitizing the plants, but also by transmitting plant viruses to some crops. Associations of corn yield loss (Norton et al., 1978), reduction of growth of shelterbelt trees (Malek and Smolik, 1975), and decline of apple (Malus domestica) trees (Wallace and MacDonald, 1979) with Xiphinema americanum have been reported from Minnesota and the neighboring states. It is possible that in some organically farmed fields in this survey the high population densities (e.g., more than 100 nematodes/100 cm3 soil) of Xiphinema may be able to cause significant yield loss to some crops.
Root-knot nematodes generally occur in warm regions and they are not common in the cold climate in Minnesota. In the previous statewide surveys conducted in 1950s, root-knot nematodes were encountered at a low frequency, but the species was not identified (Taylor et al., 1958; Taylor and Schleder, 1959). In later studies, northern root-knot nematode, Meloidogyne hapla, was observed in some fields (Crow and MacDonald, 1978), and this nematode can cause damage to some crops, such as carrot (Daucus carota) (Gugino et al., 2006), in the state. The root-knot nematode observed in this study, however, was not M. hapla based on preliminary morphological observation. Further studies are needed to identify the nematode to species and determine its economic importance.
Helicotylenchus spp., the spiral nematodes, are the most prevalent plant-parasitic nematodes in the organically farmed fields in Minnesota. The economic importance of the nematodes has not been well studied, although H. pseudorobustus, which is probably the most common species of the genus in the North Central USA, is considered a mild pathogen (Norton, 1977; Norton et al., 1978).
Tylenchorhynchus group of nematodes is taxonomically a complex of genera. Some genera included in this group found in this survey were probably Merlinius, Trophorus, and etc. These nematodes are generally considered a mild pathogen, but a high population density of T. claytoni caused significant yield loss of soybean in a microplot experiment (Ross et al., 1967). Trophorus minnesotensis was reported to associate with sugar beet (Beta vulgaris) in Minnesota, but its economic importance has not been determined (Caveness, 1958)
Other nematodes including Aphelenchoides, Hoplolaimus, and Trichodorus were encountered at a low frequency. They may have low economic significance statewide, but can be important in a specific field. Mesocriconema was encountered at low frequency in the organically farmed fields in Minnesota. This nematode has low pathogenicity to soybean and corn (Barker et al., 1982), the common crops in Minnesota, although damage to fruit trees has been reported (Braun et al., 1975; Nyczepir, 1990). The nematode probably has little economic importance in Minnesota. Paratylenchus was encountered at a relatively high frequency, but the pathogenicity of the nematode to the crops included in this study has not been established, while study has demonstrated that this nematode also can damage fruit trees (Braun et al., 1975).
Significant correlations between soil parameters examined with the abundance of plant-parasitic nematodes were only detected between numbers of Pratylenchus and soil texture and numbers of Xiphinema and soil pH. Our study showed that Pratylenchus may be more abundant in the soil with less sand, and the result is similar to the previous study in Minnesota (Wallace et al., 1993). In our study, Xiphinema was more abundant in soil with a lower pH than in soil with a higher pH within the range of pH 5.7-7.9. In contrast, Norton and Hoffmann (1974) reported that X. americanum population density increased with increasing pH from 4.5 to 7.4 and decreased with further increase of pH, while X. chambersi decreased with increasing pH from 4.5 to 6.4. Further studies are needed to determine the soil pH effect on individual species of Xiphinema.
In this study the plant-parasitic nematode communities in organically farmed fields in four regions in Minnesota and their relationship with soil factors were assessed. Our results showed that a number of plant-parasitic nematodes occur in organically farmed fields, and the population densities of some nematodes such as soybean cyst, lesion, and dagger nematodes may be high enough to cause significant yield loss to certain crops. Although most genera of the plant-parasitic nematodes were detected in all four regions sampled, the similarity of the plant-parasitic nematode community decreased with increasing distance between regions. Further studies are needed to estimate yield loss caused by the important nematode species and develop management strategies in the rapidly increasing organic-farming hectarage in the state and region.
Literature cited
- Barker KR, Schmitt DP, Campos VP. Response of peanut, corn, tobacco, and soybean to Criconemella ornata. Journal of Nematology. 1982;14:576–581. [PMC free article] [PubMed] [Google Scholar]
- Bhan M, McSorley R, Chase CA. Effect of cropping system complexity on plant-parasitic nematodes associated with organically grown vegetables in Florida. Nematropica. 2010;40:53–70. [Google Scholar]
- Birkhofer K, Bezemer TM, Bloem J, Bonkowski M, Christensen S, Dubois D, Ekelund F, Fliessbach A, Gunst L, Hedlund K, Mader P, Mikola J, Robin C, Setala H, Tatin-Froux F, Van der Putten WH, Scheu S. Long-term organic farming fosters below and aboveground biota: Implications for soil quality, biological control and productivity. Soil Biology & Biochemistry. 2008;40:2297–2308. [Google Scholar]
- Braun AL, Mojtahedi H, Lownsbery BF. Separate and combined effects of Paratylenchus neoamblycephalus and Criconemoides xenoplax on ‘Myrobalan’ plum. Phytopathology. 1975;65:328–330. [Google Scholar]
- Bray JR, Curtis JT. An ordination of the upland forest communities of southern Wisconsin. Ecological Monographs. 1957;27:325–349. [Google Scholar]
- Briar SS, Grewal PS, Somasekhar N, Stinner D, Miller SA. Soil nematode community, organic matter, microbial biomass and nitrogen dynamics in field plots transitioning from conventional to organic management. Applied Soil Ecology. 2007;37:256–266. [Google Scholar]
- Caveness FE. Clavaurotylenchus minnesotensis, n. gen., n. sp. (Tylenchinae: Nematoda) from Minnesota. Proceedings of the Helminthological Society of Washington. 1958;25:122–124. [Google Scholar]
- Chen S. 2011. Soybean cyst nematode management guide. University of Minnesota Extension. Web/URL: http://www1.extension.umn.edu/agriculture/soybean/soybean-cyst-nematode/. Accessed Auguest 22. 2012.
- Chen SY, Porter PM, Orf JH, Reese CD, Stienstra WC, Young ND, Walgenbach DD, Schaus PJ, Arlt TJ, Breitenbach FR. Soybean cyst nematode population development and associated soybean yields of resistant and susceptible cultivars in Minnesota. Plant Disease. 2001a;85:760–766. doi: 10.1094/PDIS.2001.85.7.760. [DOI] [PubMed] [Google Scholar]
- Chen SY, Porter PM, Reese CD, Stienstra WC. Crop sequence effects on soybean cyst nematode and soybean and corn yields. Crop Science. 2001b;41:1843–1849. [Google Scholar]
- Crow RV, MacDonald DH. Phytoparasitic nematodes adjacent to established strawberry plantations. Journal of Nematology. 1978;10:204–207. [PMC free article] [PubMed] [Google Scholar]
- Delate, K. 2003. Fundmental of organic agriculture. Iowa Steate University Extension. Web/URL: http://www.extension.iastate.edu/Publications/PM1880.pdf.
- Gugino BK, Abawi GS, Ludwig JW. Damage and management of Meloidogyne hapla using oxamyl on carrot in New York. Journal of Nematology. 2006;38:483–490. [PMC free article] [PubMed] [Google Scholar]
- Holtzmann OV, Aragaki M. Clover cyst nematode in Hawaii. Plant Diseases Reporter. 1963;47:889. [Google Scholar]
- Hooper DJ. 1986 Handling, fixing, staining and mounting nematodes. Pp. 59–80 in J. F. Southey, ed. Laboratory methods for work with plant and soil nematodes. London: Her Majesty's Stationery Office. [Google Scholar]
- Jenkins WR. A rapid centrifugal-flotation technique for separating nematodes from soil. Plant Disease Reporter. 1964;48:692. [Google Scholar]
- MacDonald DH. Plant-parasitic nematodes associated with field crops grown in monoculture in Minnesota. Journal of Nematology. 1979;11:306. [Google Scholar]
- MacDonald DH, Noel GR, Lueschen WE. Soybean cyst nematode, Heterodera glycines, in Minnesota. Plant Disease. 1980;64:319–321. [Google Scholar]
- MacGuidwin AE, Grau CR, Oplinger ES. Impact of planting ‘Bell’, a soybean cultivar resistant to Heterodera glycines, in Wisconsin. Journal of Nematology. 1995;27:78–85. [PMC free article] [PubMed] [Google Scholar]
- Mai WF, Bloom JR, Chen TA. 1977 Biology and ecology of the plant-parasitic nematode Pratylenchus penetrans. Pennsylvania State University, Agricultural Experiment Station Bulletin No.815. Pennsylvania: Pennsylvania State University. 65 pp. [Google Scholar]
- Malek RB, Smolik JD. Effect of Xiphinema americanum on growth of shelterbelt trees. Plant Disease Reporter. 1975;59:144–148. [Google Scholar]
- Monson M, Schmitt DP. 2004 Economics. Pp. 41–53 in D. P. Schmitt, J. A. Wrather, and R. D. Riggs, eds. Biology and management of the soybean cyst nematode. Marceline, MO: Schmitt & Associates of Marceline. [Google Scholar]
- Moyniham, M. 2010. Status of organic agriculture in Minnesota: A report to the Minnesota Legislature 2010. Minnesota Department of Agriculture. Web/URL: http://www.mda.state.mn.us/∼/media/Files/news/govrelations/organicstatusreport.ashx.
- Niblack TL. Soybean cyst nematode management reconsidered. Plant Disease. 2005;89:1020–1026. doi: 10.1094/PD-89-1020. [DOI] [PubMed] [Google Scholar]
- Niblack TL, Chen SY. 2004 Cropping systems. Pp. 181–206 in D. P. Schmitt, J. A. Wrather, and R. D. Riggs, eds. Biology and management of the soybean cyst nematode. Marceline, MO: Schmitt & Associates of Marceline. [Google Scholar]
- Norton DC. Helicotylenchus pseudorobustus as a pathogen on corn, and its densities on corn and soybean. Iowa State Journal of Research. 1977;51:279–285. [Google Scholar]
- Norton DC. 1978 Ecology of plant-parasitic nematodes. New York: Wiley-Interscience. [Google Scholar]
- Norton DC, Hoffmann JK. Distribution of selected plant parasitic nematodes relative to vegetation and edaphic factors. Journal of Nematology. 1974;6:81–86. [PMC free article] [PubMed] [Google Scholar]
- Norton DC, tollefson J, Hinz P, Thomas SH. Corn yield increases relative to nonfumigant chemical control of nematodes. Journal of Nematology. 1978;10:160–166. [PMC free article] [PubMed] [Google Scholar]
- Nyczepir AP. Influence of Criconemella xenoplax and pruning time on short life of peach trees. Journal of Nematology. 1990;22:97–100. [PMC free article] [PubMed] [Google Scholar]
- Porter PM, Chen S. Sugarbeet cyst nematode not detected in the Red River Valley of Minnesota and North Dakota. Journal of Sugar Beet Research. 2005;42:31–37. [Google Scholar]
- Powelson ML, Rowe RC. Biology and management of early dying of potatoes. Annual Review of Phytopathology. 1993;31:111–126. doi: 10.1146/annurev.py.31.090193.000551. [DOI] [PubMed] [Google Scholar]
- Riggs RD, Hamblen ML. Soybean-cyst nematode host studies in the family Leguminosae. Arkansas Agricultural Experiment Station Report Series. 1962;110:1–17. [Google Scholar]
- Riggs RD, Hamblen ML. Further studies on the host range of the soybean-cyst nematode. Arkansas Agricultural Experiment Station Bulletin. 1966;718:1–18. [Google Scholar]
- Robbins RT. Distribution of Xiphinema americanum and related species in North America. Journal of Nematology. 1993;25:344–348. [PMC free article] [PubMed] [Google Scholar]
- Ross JP, Nusbaum CJ, Hirschmann H. Soybean yield reduction by lesion, stunt, and spiral nematodes. Phytopathology. 1967;54:1128–1231. [Google Scholar]
- Rowe RC, Powelson ML. Potato early dying: Management challenges in a changing production environment. Plant Disease. 2002;86:1184–1193. doi: 10.1094/PDIS.2002.86.11.1184. [DOI] [PubMed] [Google Scholar]
- Siddiqi MR. 2000 Tylenchida: Parasites of plants and insects, second edition. New York: CAB International. [Google Scholar]
- Sohlenius B, Persson H, Magnusson C. Distribution of roots and nematodes in a young Scots pine stand in Central Sweden. Ecological Bulletin (Stockholm) 1977;25:340–347. [Google Scholar]
- Spears JF. Occurrence of the grass cyst nematode, Heterodera punctata and Heterodera cacti group cysts in North Dakota and Minnesota. Plant Disease Reporter. 1956;40:583–584. [Google Scholar]
- Taylor DP, Anderson RV, Haglund WA. Nematodes associated with Minnesota crops. I. Preliminary survey of nematodes associated with alfalfa, flax, peas, and soybeans. Plant Disease Reporter. 1958;42:195–198. [Google Scholar]
- Taylor DP, Schleder EG. Nematodes associated with Minnesota crops. II. Nematodes associated with corn, barley, oats, rye, and wheat. Plant Disease Reporter. 1959;43:329–333. [Google Scholar]
- USDA. 2012. Organic certification. Available at: http://www.usda.gov/wps/portal/usda/usdahome?navid=ORGANIC_CERTIFICATIO. Accessed 23 August, 2012.
- Wallace MK, MacDonald DH. Plant-parasitic nematodes in Minnesota apple orchards. Plant Disease Reporter. 1979;63:1063–1067. [Google Scholar]
- Wallace MK, Rust RH, Hawkins DM, MacDonald DH. Correlation of edaphic factors with plant-parasitic nematode population densities in a forage-field. Journal of Nematology. 1993;25:642–653. [PMC free article] [PubMed] [Google Scholar]
- Wrather JA, Koenning SR. Estimates of disease effects on soybean yields in the United States 2003 to 2005. Journal of Nematology. 2006;38:173–180. [PMC free article] [PubMed] [Google Scholar]
- Yeates GW, Bongers T, De Goede RGM, Freckman DW, Georgieva SS. Feeding habits in soil nematode families and genera - an outline for soil ecologists. Journal of Nematology. 1993;25:315–331. [PMC free article] [PubMed] [Google Scholar]
- Young LD. Influence of soybean cropping sequences on seed yield and female index of the soybean cyst nematode. Plant Disease. 1998;82:615–619. doi: 10.1094/PDIS.1998.82.6.615. [DOI] [PubMed] [Google Scholar]



