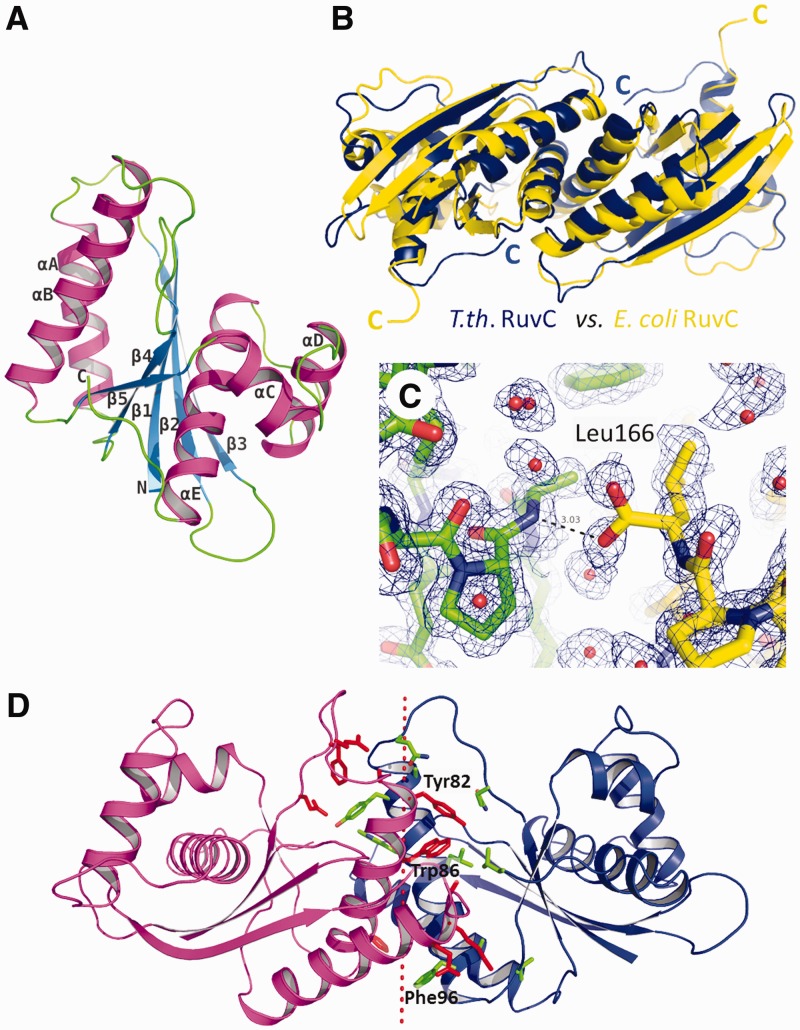
Figure 2.
Structure of T.th. RuvC. (A) T.th. RuvC monomer shown in a ribbon diagram, with the secondary structure elements labeled. (B) T.th. RuvC dimer in blue superimposed on the E. coli RuvC dimer in yellow. The α-helices are packed more tightly at the dimer interface in the T.th. RuvC dimer, with the C-termini of the proteins tucked in. (C) The C-terminal residue Leu166 of T.th. RuvC is well ordered, with the carboxyl group involved in hydrogen bonding across the dimer interface. The two different molecules within the T.th. RuvC dimer are colored differently (yellow and green) to highlight the intermolecular interaction. The simulated annealing composite omit 2Fo-Fc map is contoured at 1.0σ. (D) Residues involved in the dimer interface are shown, with the aromatic residues Tyr82, Trp86 and Phe96 highlighted. The dotted line represents the pseudo 2-fold axis relating the two molecules in the T.th. RuvC dimer.