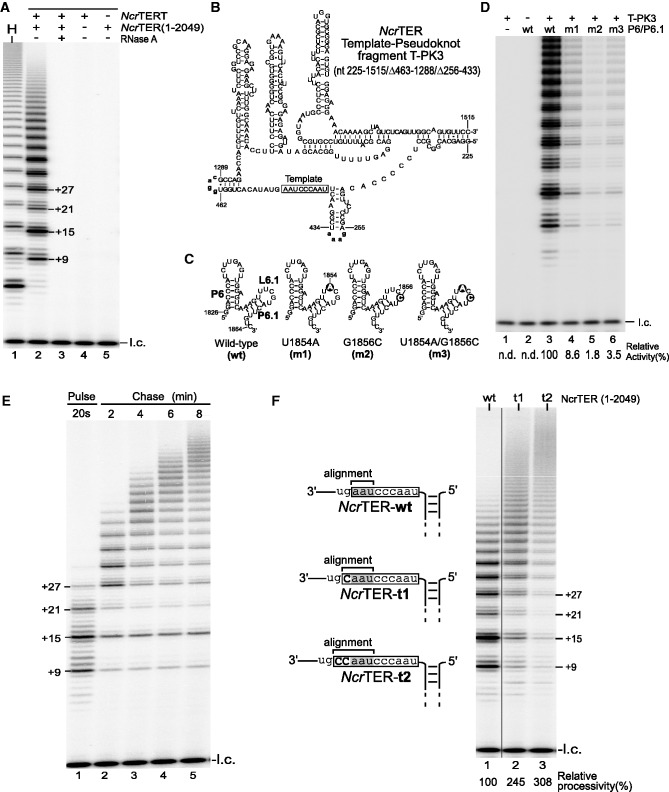
Figure 5.
Functional characterization of N. crassa telomerase activity. (A) Direct primer-extension assay of in vitro-reconstituted N. crassa telomerase. Recombinant NcrTERT protein was synthesized in RRL and assembled with 0.1 µM full-length 2049-nt NcrTER. The reconstituted N. crassa telomerase was analyzed by direct primer-extension assay using the telomeric primer (TTAGGG)3. The major bands (+9, +15, +21 and +27) are denoted on the right of lane 2, indicating the number of nucleotides added to the primer. Addition of RNase A (lane 3), the absence of NcrTER (lane 4) and the absence of NcrTERT (lane 5) is indicated above the gel. Human telomerase (H) served as a positive control (lane 1). A 32P-end-labeled 15-mer oligonucleotide was included as loading control (l.c.) before purification and precipitation of telomerase-extended products. (B) Secondary structure of the minimal 295-nt NcrTER template–pseudoknot fragment T-PK3. The T-PK3 fragment contains nucleotides 225–1515, with two internal deletions (nt 256–433 and 463–1288) replaced with tetraloops (bold, lower case), GAAA and GGAC, respectively. The template region is denoted by an open box. (C) The 39-nt NcrTER P6/6.1 RNA fragments with L6.1 mutations. Point mutations, U1854A (m1), G1856C (m2) and U1854A/G1856C (m3), in the L6.1 loop are indicated by solid circles (D). Mutations in loop L6.1 severely reduced telomerase activity. N. crassa telomerase was reconstituted in RRL from the recombinant NcrTERT protein and two separate RNA fragments, T-PK3 and P6/6.1, and were analyzed by direct primer-extension assay. The RNA fragments assayed within each reaction are denoted above the gel. Relative telomerase activity is shown under the gel (lane 4–6) and was determined by normalizing the total intensities of all bands within each lane to wild-type activity in lane 3. The relative activity was not determined (n.d.) for lanes 1 and 2. A 32P-end-labeled 15-mer oligonucleotide was included as the loading control (l.c.). (E) Pulse-chase time course analysis of N. crassa telomerase. In vitro-reconstituted telomerase was incubated with telomeric primer (TTAGGG)3 for 20 s at room temperature in the presence of radioactive α-32P-dGTP, dTTP and dATP. An aliquot of the reaction was removed and terminated after 20 s to determine the initial length of the product (lane 1). Chase reaction was initiated by adding 50 folds of non-radioactive dGTP and 10 folds of competitive DNA oligonucletide 5′-(TTAGGG)3-3′-amine. Aliquots of the chase reaction was removed and terminated after 2, 4, 6 or 8 min from the start of the chase reaction (lane 2–5). A 15-mer 32P-end-labeled oligonucleotide was added as loading control (l.c.). (F) Effect of template length on repeat addition processivity. Full-length NcrTER (1–2049) with either the wild-type 9-nt template (wt), 10-nt template (t1) or 11-nt template (t2) was reconstituted with NcrTERT protein in RRL and analyzed by direct primer-extension assay. The NcrTER template sequence is denoted by an open box, with the alignment region shaded. Relative processivity was determined by normalization to wild-type processivity and is shown below the gel. A 15-mer 32P-end-labeled oligonucleotide was included as loading control (l.c.).