Figure 9.
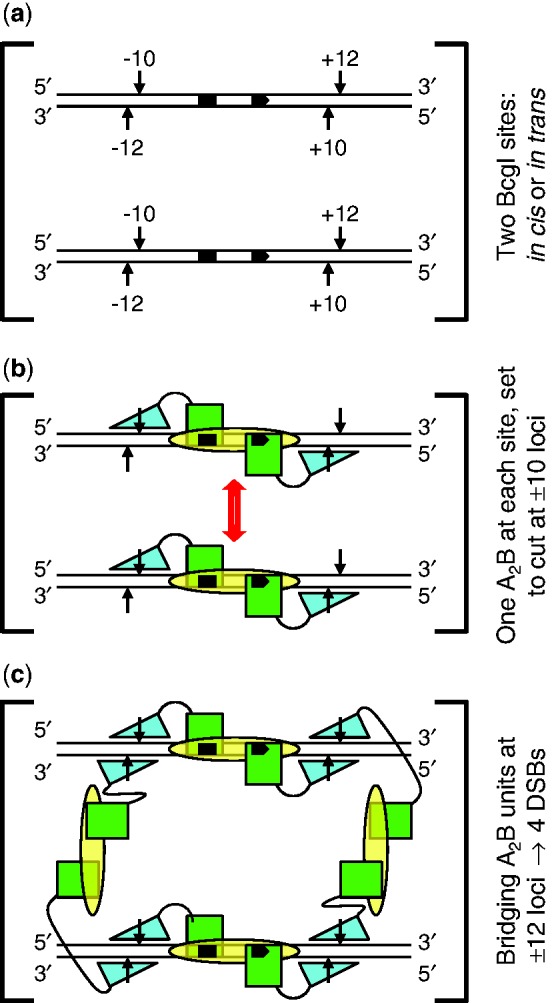
Scheme for action at eight phosphodiester bonds by BcgI. (a) The substrate for the REase activity of BcgI consists of two copies of its recognition sequence. The sites, shown here aligned in parallel, can be located either on the same DNA molecule, in cis, or on separate molecules, in trans. The bipartite recognition site is indicated by black segments, with an arrowhead to mark its 5′–3′ orientation: strand polarities are also indicated at DNA termini. Cleavage loci 10 and 12 nt distant from the site are marked with vertical arrows: upstream and downstream loci are noted with − or + signs, respectively. (b) The two sites in (a) are both shown bound to one A2B protomer of BcgI: an interaction between the protomers is suggested by the double-headed arrow (in red). The B subunit (yellow oval) is responsible for DNA specificity and is positioned spanning both segments of the recognition sequence. The A subunits carry both REase and MTase activities and are illustrated as separate domains connected by a flexible linker. The MTase domains (green squares) overlay the sites of methylation in the specified segments of the recognition sequence. The REase domains (cyan triangles) are placed against the target bonds at the −10 and +10 positions, upstream and downstream of the site in top and bottom strands, respectively: to mark their orientation on the DNA, the triangle points towards the 5′-end of each strand. At this stage, BcgI has yet to engage the scissile bonds at the −12 and +12 positions. (c) As (b) except that two additional A2B units from free solution now bridge the A2B units bound to each site via interactions between the nuclease domains of the free and the DNA-bound units. The two nuclease domains from one of the extra A2B units engage the scissile bonds at the −12 positions upstream of both recognition sites (on the left, as drawn in [c]) while those from the other occupy the downstream +12 positions in both sites (on the right). The scheme thus results in a dimer of nuclease domains, with two catalytic centres for phosphodiester hydrolysis at all four loci where BcgI makes a DSB.
