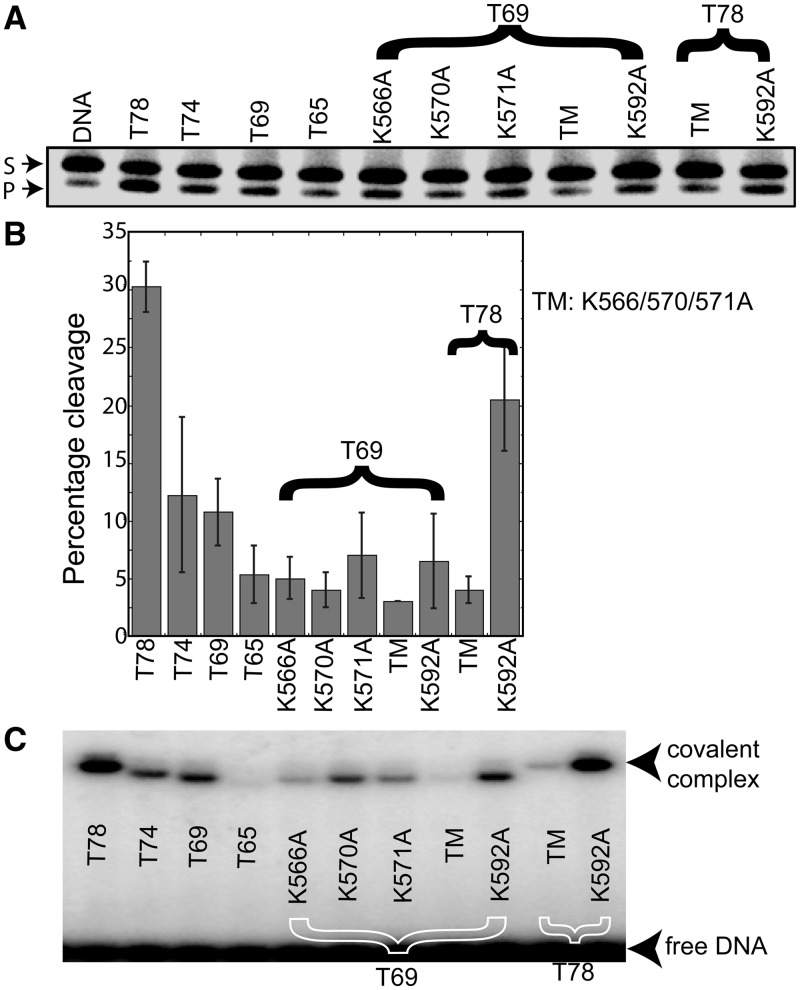
Figure 2.
dRP lyase assay with the different Topo-V fragments. (A) A 34-bp DNA containing a single U was pre-treated with UDG to generate an abasic site. The AP site-containing DNA was further treated with AP endonuclease to incise the DNA at the abasic site and used as the substrate for dRP lyase activity with different Topo-V fragments, as indicated. The lower band on the gel shows the product (P) after removal of the sugar phosphate at the abasic site, whereas the upper band illustrates the substrate (S). (B) Graph illustrating the quantification of the dRP lyase activity (%) of different Topo-V fragments. The results represent averages from four independent experiments. Comparison of lysine mutants on the Topo-69 and Topo-78 backbones shows that K566, K570 and K571 are required for dRP lyase activity, whereas K592 is not. Error bars represent the standard deviation for four replications. (C) NaBH4 cross-linking of the different Topo-V fragments with DNA. Topo-V fragments were incubated with abasic DNA and 1 mM NaBH4 to trap the Schiff base protein–DNA intermediate, as described under ‘Materials and Methods’ section. The results show that there is no DNA–protein complex formation for either the Topo-65 or the Lys triple mutant (K566A/570A/571A), although the single Lys mutants do form a DNA–protein complex. TM refers to the K566A, K570A, K571A triple mutant.