Abstract
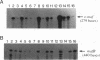
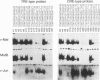
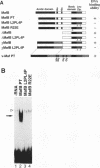
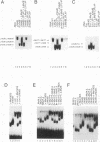
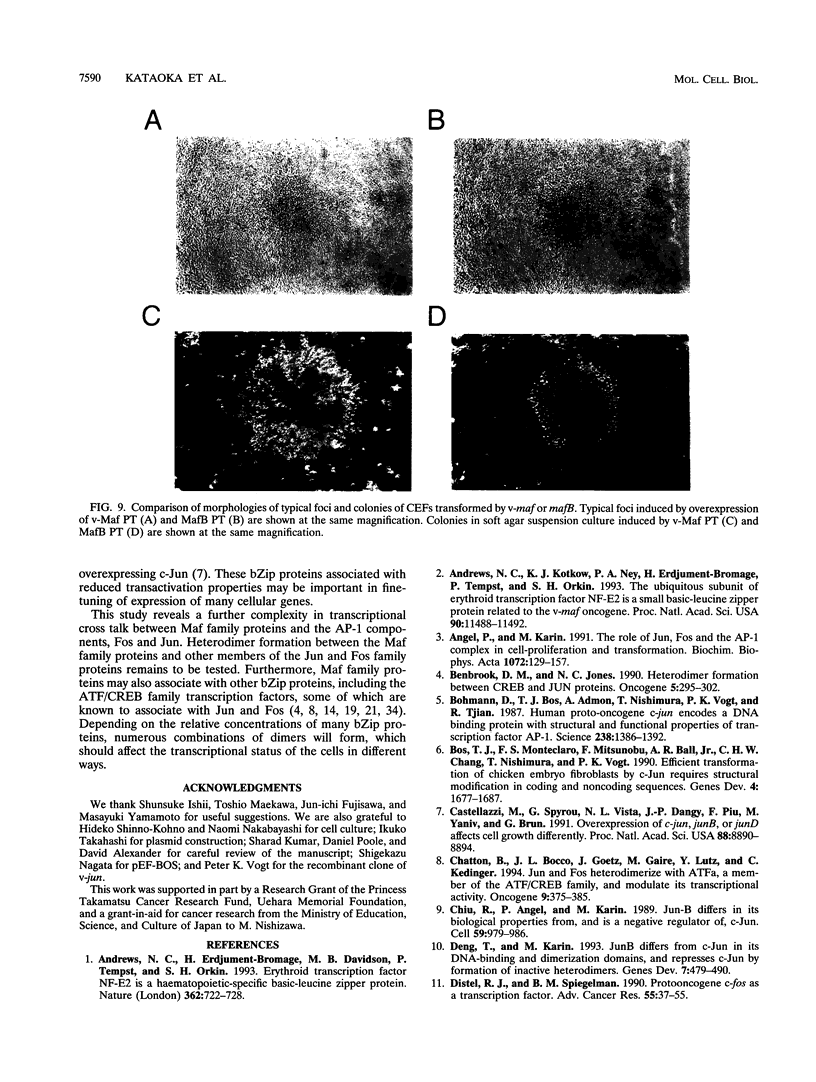
We have identified a new member of the maf oncogene family and named it mafB. This gene is expressed in a wide variety of tissues and encodes a protein of 311 amino acids containing a typical bZip motif in its carboxy-terminal region. In the bZip domain, MafB shares extensive homology not only with v-Maf but also with other Maf-related proteins. As expected from its structure, MafB forms a homodimer through its leucine repeat structure and specifically binds Maf-recognition elements (MAREs). In addition, MafB forms heterodimers with v-Maf and Fos through its zipper structure. However, unlike v-Maf, MafB fails to associate with Jun. Transient cotransfection assays revealed that both v-Maf and MafB act as transactivators for a promoter linked to MAREs, although MafB is less potent than v-Maf. As is the case for the c-maf gene, overexpression of the mafB gene induces transformation of chicken embryo fibroblasts in vitro. Through formation of numerous bZip dimers, the Maf family proteins along with the AP-1 components should provide great diversity in transcriptional regulation for a wide variety of genes.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews N. C., Erdjument-Bromage H., Davidson M. B., Tempst P., Orkin S. H. Erythroid transcription factor NF-E2 is a haematopoietic-specific basic-leucine zipper protein. Nature. 1993 Apr 22;362(6422):722–728. doi: 10.1038/362722a0. [DOI] [PubMed] [Google Scholar]
- Andrews N. C., Kotkow K. J., Ney P. A., Erdjument-Bromage H., Tempst P., Orkin S. H. The ubiquitous subunit of erythroid transcription factor NF-E2 is a small basic-leucine zipper protein related to the v-maf oncogene. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11488–11492. doi: 10.1073/pnas.90.24.11488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel P., Karin M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim Biophys Acta. 1991 Dec 10;1072(2-3):129–157. doi: 10.1016/0304-419x(91)90011-9. [DOI] [PubMed] [Google Scholar]
- Benbrook D. M., Jones N. C. Heterodimer formation between CREB and JUN proteins. Oncogene. 1990 Mar;5(3):295–302. [PubMed] [Google Scholar]
- Bohmann D., Bos T. J., Admon A., Nishimura T., Vogt P. K., Tjian R. Human proto-oncogene c-jun encodes a DNA binding protein with structural and functional properties of transcription factor AP-1. Science. 1987 Dec 4;238(4832):1386–1392. doi: 10.1126/science.2825349. [DOI] [PubMed] [Google Scholar]
- Bos T. J., Monteclaro F. S., Mitsunobu F., Ball A. R., Jr, Chang C. H., Nishimura T., Vogt P. K. Efficient transformation of chicken embryo fibroblasts by c-Jun requires structural modification in coding and noncoding sequences. Genes Dev. 1990 Oct;4(10):1677–1687. doi: 10.1101/gad.4.10.1677. [DOI] [PubMed] [Google Scholar]
- Castellazzi M., Spyrou G., La Vista N., Dangy J. P., Piu F., Yaniv M., Brun G. Overexpression of c-jun, junB, or junD affects cell growth differently. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):8890–8894. doi: 10.1073/pnas.88.20.8890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatton B., Bocco J. L., Goetz J., Gaire M., Lutz Y., Kedinger C. Jun and Fos heterodimerize with ATFa, a member of the ATF/CREB family and modulate its transcriptional activity. Oncogene. 1994 Feb;9(2):375–385. [PubMed] [Google Scholar]
- Chiu R., Angel P., Karin M. Jun-B differs in its biological properties from, and is a negative regulator of, c-Jun. Cell. 1989 Dec 22;59(6):979–986. doi: 10.1016/0092-8674(89)90754-x. [DOI] [PubMed] [Google Scholar]
- Deng T., Karin M. JunB differs from c-Jun in its DNA-binding and dimerization domains, and represses c-Jun by formation of inactive heterodimers. Genes Dev. 1993 Mar;7(3):479–490. doi: 10.1101/gad.7.3.479. [DOI] [PubMed] [Google Scholar]
- Distel R. J., Spiegelman B. M. Protooncogene c-fos as a transcription factor. Adv Cancer Res. 1990;55:37–55. doi: 10.1016/s0065-230x(08)60467-4. [DOI] [PubMed] [Google Scholar]
- Dunn I. S., Blattner F. R. Charons 36 to 40: multi enzyme, high capacity, recombination deficient replacement vectors with polylinkers and polystuffers. Nucleic Acids Res. 1987 Mar 25;15(6):2677–2698. doi: 10.1093/nar/15.6.2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara K. T., Kataoka K., Nishizawa M. Two new members of the maf oncogene family, mafK and mafF, encode nuclear b-Zip proteins lacking putative trans-activator domain. Oncogene. 1993 Sep;8(9):2371–2380. [PubMed] [Google Scholar]
- Hai T., Curran T. Cross-family dimerization of transcription factors Fos/Jun and ATF/CREB alters DNA binding specificity. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3720–3724. doi: 10.1073/pnas.88.9.3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartl M., Hutchins J. T., Vogt P. K. The chicken junD gene and its product. Oncogene. 1991 Sep;6(9):1623–1631. [PubMed] [Google Scholar]
- Hartl M., Vogt P. K. A rearranged junD transforms chicken embryo fibroblasts. Cell Growth Differ. 1992 Dec;3(12):909–918. [PubMed] [Google Scholar]
- Hartl M., Vogt P. K. Oncogenic transformation by Jun: role of transactivation and homodimerization. Cell Growth Differ. 1992 Dec;3(12):899–908. [PubMed] [Google Scholar]
- Hirai S. I., Ryseck R. P., Mechta F., Bravo R., Yaniv M. Characterization of junD: a new member of the jun proto-oncogene family. EMBO J. 1989 May;8(5):1433–1439. doi: 10.1002/j.1460-2075.1989.tb03525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu J. C., Laz T., Mohn K. L., Taub R. Identification of LRF-1, a leucine-zipper protein that is rapidly and highly induced in regenerating liver. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3511–3515. doi: 10.1073/pnas.88.9.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi K., Kataoka K., Itoh K., Hayashi N., Nishizawa M., Yamamoto M. Regulation of transcription by dimerization of erythroid factor NF-E2 p45 with small Maf proteins. Nature. 1994 Feb 10;367(6463):568–572. doi: 10.1038/367568a0. [DOI] [PubMed] [Google Scholar]
- Ivashkiv L. B., Liou H. C., Kara C. J., Lamph W. W., Verma I. M., Glimcher L. H. mXBP/CRE-BP2 and c-Jun form a complex which binds to the cyclic AMP, but not to the 12-O-tetradecanoylphorbol-13-acetate, response element. Mol Cell Biol. 1990 Apr;10(4):1609–1621. doi: 10.1128/mcb.10.4.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadonaga J. T., Carner K. R., Masiarz F. R., Tjian R. Isolation of cDNA encoding transcription factor Sp1 and functional analysis of the DNA binding domain. Cell. 1987 Dec 24;51(6):1079–1090. doi: 10.1016/0092-8674(87)90594-0. [DOI] [PubMed] [Google Scholar]
- Kataoka K., Nishizawa M., Kawai S. Structure-function analysis of the maf oncogene product, a member of the b-Zip protein family. J Virol. 1993 Apr;67(4):2133–2141. doi: 10.1128/jvi.67.4.2133-2141.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka K., Noda M., Nishizawa M. Maf nuclear oncoprotein recognizes sequences related to an AP-1 site and forms heterodimers with both Fos and Jun. Mol Cell Biol. 1994 Jan;14(1):700–712. doi: 10.1128/mcb.14.1.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai S., Goto N., Kataoka K., Saegusa T., Shinno-Kohno H., Nishizawa M. Isolation of the avian transforming retrovirus, AS42, carrying the v-maf oncogene and initial characterization of its gene product. Virology. 1992 Jun;188(2):778–784. doi: 10.1016/0042-6822(92)90532-t. [DOI] [PubMed] [Google Scholar]
- Kawai S., Koyama T. Characterization of a Rous sarcoma virus mutant defective in packaging its own genomic RNA: biological properties of mutant TK15 and mutant-induced transformants. J Virol. 1984 Jul;51(1):147–153. doi: 10.1128/jvi.51.1.147-153.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai S., Nishizawa M. New procedure for DNA transfection with polycation and dimethyl sulfoxide. Mol Cell Biol. 1984 Jun;4(6):1172–1174. doi: 10.1128/mcb.4.6.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerppola T. K., Curran T. Maf and Nrl can bind to AP-1 sites and form heterodimers with Fos and Jun. Oncogene. 1994 Mar;9(3):675–684. [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Landschulz W. H., Johnson P. F., McKnight S. L. The leucine zipper: a hypothetical structure common to a new class of DNA binding proteins. Science. 1988 Jun 24;240(4860):1759–1764. doi: 10.1126/science.3289117. [DOI] [PubMed] [Google Scholar]
- Macgregor P. F., Abate C., Curran T. Direct cloning of leucine zipper proteins: Jun binds cooperatively to the CRE with CRE-BP1. Oncogene. 1990 Apr;5(4):451–458. [PubMed] [Google Scholar]
- Mellström B., Achaval M., Montero D., Naranjo J. R., Sassone-Corsi P. Differential expression of the jun family members in rat brain. Oncogene. 1991 Nov;6(11):1959–1964. [PubMed] [Google Scholar]
- Mizushima S., Nagata S. pEF-BOS, a powerful mammalian expression vector. Nucleic Acids Res. 1990 Sep 11;18(17):5322–5322. doi: 10.1093/nar/18.17.5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakabeppu Y., Ryder K., Nathans D. DNA binding activities of three murine Jun proteins: stimulation by Fos. Cell. 1988 Dec 2;55(5):907–915. doi: 10.1016/0092-8674(88)90146-8. [DOI] [PubMed] [Google Scholar]
- Nishizawa M., Kataoka K., Goto N., Fujiwara K. T., Kawai S. v-maf, a viral oncogene that encodes a "leucine zipper" motif. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7711–7715. doi: 10.1073/pnas.86.20.7711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfarr C. M., Mechta F., Spyrou G., Lallemand D., Carillo S., Yaniv M. Mouse JunD negatively regulates fibroblast growth and antagonizes transformation by ras. Cell. 1994 Feb 25;76(4):747–760. doi: 10.1016/0092-8674(94)90513-4. [DOI] [PubMed] [Google Scholar]
- Ryder K., Lanahan A., Perez-Albuerne E., Nathans D. jun-D: a third member of the jun gene family. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1500–1503. doi: 10.1073/pnas.86.5.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder K., Lau L. F., Nathans D. A gene activated by growth factors is related to the oncogene v-jun. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1487–1491. doi: 10.1073/pnas.85.5.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryseck R. P., Bravo R. c-JUN, JUN B, and JUN D differ in their binding affinities to AP-1 and CRE consensus sequences: effect of FOS proteins. Oncogene. 1991 Apr;6(4):533–542. [PubMed] [Google Scholar]
- Schütte J., Viallet J., Nau M., Segal S., Fedorko J., Minna J. jun-B inhibits and c-fos stimulates the transforming and trans-activating activities of c-jun. Cell. 1989 Dec 22;59(6):987–997. doi: 10.1016/0092-8674(89)90755-1. [DOI] [PubMed] [Google Scholar]
- Semba K., Kawai S., Matsuzawa Y., Yamanashi Y., Nishizawa M., Toyoshima K. Transformation of chicken embryo fibroblast cells by avian retroviruses containing the human Fyn gene and its mutated genes. Mol Cell Biol. 1990 Jun;10(6):3095–3104. doi: 10.1128/mcb.10.6.3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaroop A., Xu J. Z., Pawar H., Jackson A., Skolnick C., Agarwal N. A conserved retina-specific gene encodes a basic motif/leucine zipper domain. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):266–270. doi: 10.1073/pnas.89.1.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinson C. R., Sigler P. B., McKnight S. L. Scissors-grip model for DNA recognition by a family of leucine zipper proteins. Science. 1989 Nov 17;246(4932):911–916. doi: 10.1126/science.2683088. [DOI] [PubMed] [Google Scholar]
- Vogt P. K., Bos T. J. jun: oncogene and transcription factor. Adv Cancer Res. 1990;55:1–35. doi: 10.1016/s0065-230x(08)60466-2. [DOI] [PubMed] [Google Scholar]
- Wilkinson D. G., Bhatt S., Ryseck R. P., Bravo R. Tissue-specific expression of c-jun and junB during organogenesis in the mouse. Development. 1989 Jul;106(3):465–471. doi: 10.1242/dev.106.3.465. [DOI] [PubMed] [Google Scholar]