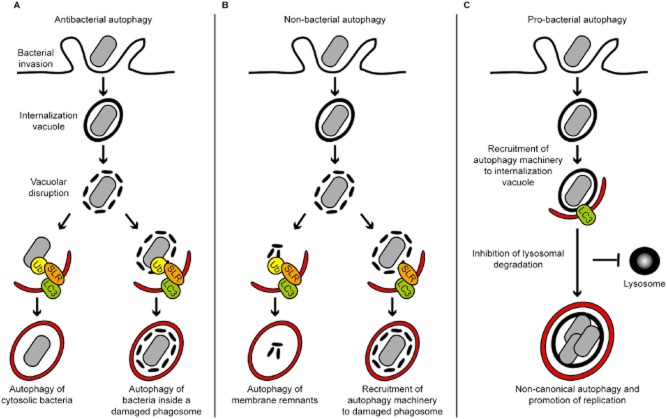
Fig. 1.
Different autophagy pathways triggered by bacterial invasion.
A. Antibacterial autophagy. After entry into host cells, bacteria are localized inside an internalization vacuole. Upon vacuolar disruption, autophagy may recognize ubiquitination signals and intracellular pathogens located (left) in the cytosol (e.g. L. monocytogenes, S. flexneri, S. Typhimurium) and (right) inside a damaged internalization vacuole (e.g. M. tuberculosis). In both cases, the enclosed bacterium is delivered to the lysosome for degradation.
B. Non-bacterial autophagy. Autophagy may be targeted against cellular disturbances arising from the bacterial invasion process, such as membrane damaged from bacterial entry or vacuolar disruption. (Left) Damaged membrane, and inflammasome components localized to damaged membrane, may be ubiquitinated and targeted to autophagy. (Right) Damaged membrane can also be recognized for autophagy by non-ubiquitin signals (e.g. NDP52–galectin 8). In both cases, non-bacterial autophagy may trigger cell autonomous signalling and influence bacterial replication.
C. Pro-bacterial autophagy. Some internalized bacteria (e.g. S. aureus, B. abortus) may recruit a subset of the autophagy machinery and create a replicative niche inside an autophagosome-like vacuole. These bacteria subvert the autophagy machinery to avoid degradation in a lysosomal compartment and support bacterial replication. Ub, ubiquitin; SLR, autophagy receptor (e.g. p62, NDP52); LC3, ATG8 family proteins.