Fig. 3. In vitro assembly of zebrafish Bfsp2 with BFSP1.
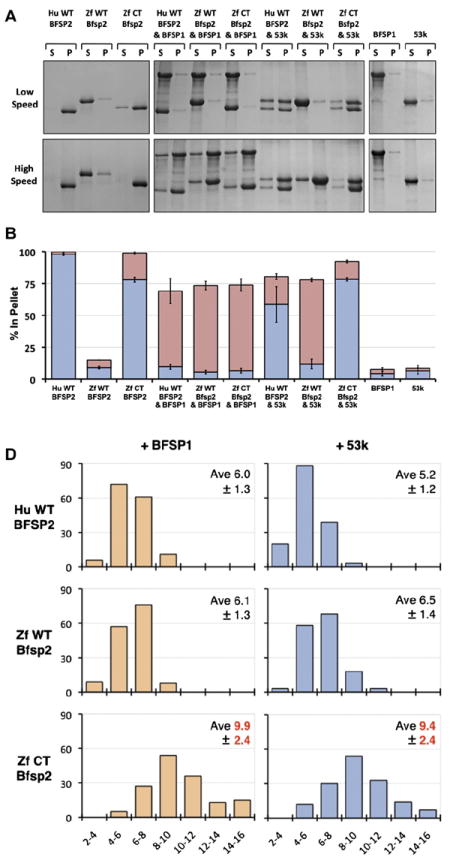
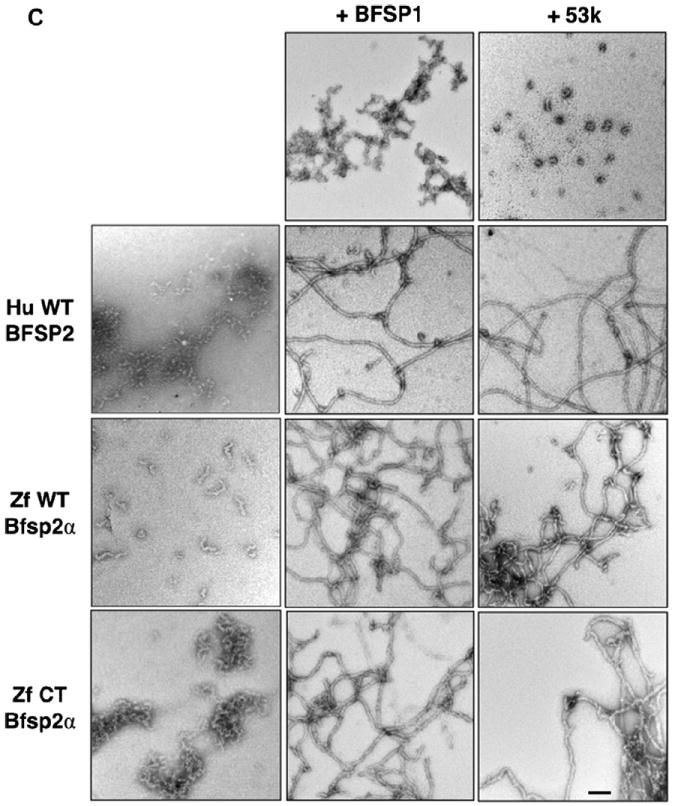
A Assembly of human (Hu WT) and zebrafish (wild-type (Zf WT) and C-terminally truncated (Zf CT)) Bfsp2 proteins with bovine native BFSP1 or bovine native BFSP1-53 kDa (53 k). Prior to assembly, the named protein combinations were mixed in 1:1 mass ratios. Assembly was analysed by low- and high-speed sedimentation assays. Representative examples from supernatant (s) and pellet (p) fractions obtained after low- and high-speed sedimentation assays are shown. By sedimentation assay, both bovine BFSP1 and its 53 kDa (53 k) fragment remained mostly (>90%) in the soluble fractions when assembled alone. Only when these proteins were combined with an appropriate assembly partner were they found in the pellet fractions. Notice that wild-type zebrafish Bfsp2α was as efficient as human wild-type BFSP2 in shifting BFSP1 and its 53 kDa fragment to the pellet fraction in the high-speed sedimentation assay (Zf WT Bfsp2α & BFSP1, red bar; Zf WT Bfsp2α & 53 k, red bar). In fact, coassembly with BFSP1 also shifted wild-type zebrafish Bfsp2α to the pellet fraction indicative of a compatible partnership for coassembly. B. Densitometric quantification of low- (blue) and high-speed (red) sedimentation data from ‘A’. The filaments formed by a combination of zebrafish wild-type Bfsp2α and bovine BFSP1-53 kDa fragment (ZfWT Bfsp2α & 53 k) self-associate less than those formed when C-terminally truncated Bfsp2α or human wild-type BFSP2 were combined with the 53 kDa fragment of BFSP1 (Zf CT Bfsp2α & 53 k and HuWT BFSP2 & 53 k respectively), as shown by the low-speed sedimentation assay. Filaments formed by a combination of zebrafish Bfsp2α and bovine BFSP1 self-associate less then those formed by a combination of zebrafish Bfsp2α and BFSP1-53 kDa (cf blue columns for Zf WT Bfsp2α & BFSP1 and Zf WT Bfsp2α & 53 k). C. Electron microscopy characterisation of the in vitro assembled combinations of zebrafish wild-type Bfsp2α (Zf WT Bfsp2α), C-terminally truncated zebrafish Bfsp2 (Zf CT Bfsp2α), human BFSP2 (Hu WT BFSP2) with either BFSP1 (BFSP1) or BFSP1-53 kDa fragment (53 k). The left hand column shows the Bfsp2 proteins assembled alone. The centre and right hand columns show the Bfsp2 proteins assembled with BFSP1 or BFSP1-53 kDa fragment respectively. The first row shows BFSP1 and BFSP1-53 kDa assembled alone. The same magnification was used for all micrographs. Bar = 100 nm. D. Effect of the zebrafish C-terminal tail sequences upon the width of in vitro assembled filaments. The left and right hand columns show filament width distributions when the Bfsp2 proteins are assembled with BFSP1 or BFSP1-53 kDa respectively. The x axis show filament width catagories (nm) and the y axis show measurement frequency. The average (Ave) and standard deviation (±) of filament widths are also shown. A total of 150 width measurements were made for each sample. Filaments formed by Zf CT Bfsp2α are significantly larger and more varied in width than those formed by Hu WT BFSP2 or Zf WT Bfsp2α. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
