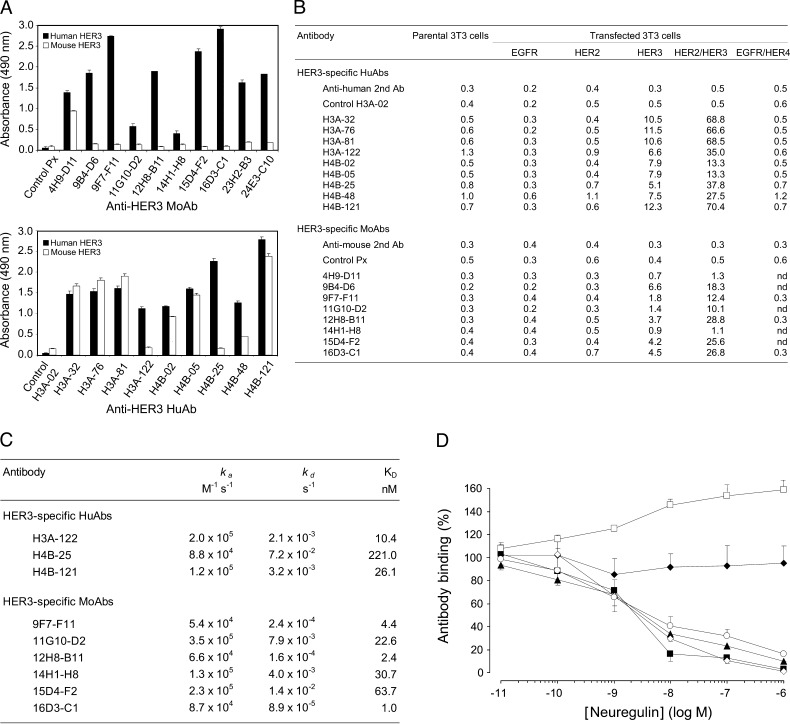
Figure 1.
Selected anti-HER3 Abs bind to the extracellular region of human HER3 but not to the other HER receptors, with or without interference with NRG binding. (A) Cross-reactivity of anti-HER3 moAbs and huAbs toward mouse or human soluble HER3 was assessed by ELISAs. The absorbance values at 490 nm of the irrelevant control Abs Px and H3A-02 are indicated. Each value represents the mean ± SD of triplicate determinations in three independent experiments. (B) Flow cytometric analysis of the anti-HER3 Ab binding to NIH/3T3 fibroblasts transfected with membrane EGFR, HER2, HER3, HER2/HER3, or EGFR/HER4 in comparison to parental NIH/3T3 cells. Binding of the FITC-conjugated secondary Abs and of the control Abs H3A-02 and Px is also indicated. Results (geometric mean) are representative of two different experiments. (C) BiaCore determination of the binding kinetics between anti-HER3 huAbs and recombinant human HER3 (3.12–6.25 nM) and between moAbs and human HER3 (extracellular)-Fc recombinant protein (77 nM), respectively. (D) NRG interference with anti-HER3 Ab binding in HER3-positive SKBR3 cells was determined by competitive flow cytometry. The binding (%) of the moAbs 9F7-F11 (□), 16D3-C1 (○), and 12H8-B11 (◊), and of the huAbs H4B-121 (▴), H3A-122 (▪), and H4B-25 (◆) to SKBR3 cells, after incubation with various NRG concentrations, is indicated. Each value represents the mean ± SD of triplicate determinations in three independent experiments. No cell binding was observed when only the FITC-conjugated secondary Ab was added after incubation with NRG.