Abstract
Hematopoietic stem cells (HSC) are maintained in a tightly regulated bone microenvironment constituted by a rich milieu of cells. Bone cells such as osteoblasts are associated with niche maintenance as regulators of the endosteal microenvironment. Bone remodeling also plays a role in HSC mobilization although it is poorly defined. The effects of zoledronic acid (ZA), a potent bisphosphonate that inhibits bone resorption, were investigated on bone marrow cell populations focusing on HSCs, and the endosteal and vascular niches in bone. ZA treatment significantly increased bone volume and HSCs in both young and adult mice (4 week and 4 month old, respectively). ZA increased vessel numbers with no overall change in vascular volume in bones of young and had no effect on vasculature in adult mice. Since both young and adult mice had increased HSCs and bone mass with differing vasculature responses, this suggests that ZA indirectly supports HSCs via the osteoblastic niche and not the vascular niche. Additionally, gene expression in Lin- cells demonstrated increased expression of self-renewal-related genes Bmi1 and Ink4a suggesting a role of ZA in the modulation of cell commitment and differentiation toward a long-term self-renewing cell. Genes that support the osteoblastic niche, BMP2 and BMP6 were also augmented in ZA treated mice. In conclusion, ZA-induced HSC expansion occurs independent of the vascular niche via indirect modulation of the osteoblastic niche.
Keywords: HEMATOPOIETIC STEM CELLS, NICHE, BONE VASCULATURE, BLOOD VESSELS, BISPHOSPHONATE, ZOLEDRONIC ACID
Bone is an important hematopoietic organ tightly regulated by the diverse cellular components that constitute its microenvironment. This unique microenvironment, also known as the stem cell niche, maintains and regulates hematopoietic stem cells (HSCs). HSCs are found in close association with the endosteal interface of bone and bone marrow, and perivascular sinusoidal blood vessels [Kiel et al., 2005; Adams et al., 2006]. Osteoblasts, endothelial cells, adipocytes and stromal cells are key cells that constitute the niche and participate in the regulation and maintenance of HSCs [Bianco, 2011]. The hematopoietic niche is necessary to balance the expansion and mobilization of HSCs to the peripheral blood as well as the regulation of self-renewal capacity and hematopoietic commitment to differentiation. Moreover, the bone microenvironment plays an important role in this regulation. For instance, studies have demonstrated that increased osteoblast number augments HSC numbers in bone marrow [Calvi et al., 2003; Zhang et al., 2003] whereas, osteoblast depletion leads to a reduction in HSC numbers [Visnjic et al., 2004]. It is not well defined whether enrichment of bone structures such as vasculature or the endosteum can alter the HSC pool in bone marrow. In this context, therapies that involve modulation of bone formation or remodeling may affect HSC expansion and mobilization.
Osteoclasts are believed to play a role in the bone microenvironment and consequently affect the HSC microenvironment. For instance, osteoclast-induced bone resorption is responsible for the release of various growth factors, proteins, and bone mineral such as calcium, all of which are known to influence in the hematopoietic cells and niche maintenance and mobilization [Adams et al., 2006; Kollet et al., 2006]. Still, the precise role of osteoclasts in HSC niche maintenance and mobilization is ill defined and controversial. Although augmented osteoclast activation results in increased stress-induced mobilization of HSCs [Cho et al., 2010], osteopetrotic animal models and pharmacological strategies of osteoclast inhibition resulted in increased mobilization of HSCs in response to G-CSF stimulation [Miyamoto et al., 2011].
Pharmacologic strategies such as bisphosphonates inhibit osteoclast-mediated bone resorption. Zoledronic acid (ZA) is a potent third generation bisphosphonate utilized for treatment of metabolic bone diseases such as osteoporosis, Paget’s disease and cancer-related bone diseases. The high affinity for hydroxyapatite results in accumulation in bone that when it is resorbed by osteoclasts inhibition of the mevalonate pathway and apoptotic cell death occurs [Rodan and Fleisch, 1996; Winter and Coleman, 2009]. However, the mechanisms of which osteoclast inhibition affects the HSC niche and progenitor cells expansion are yet unclear.
It was hypothesized that osteoclasts may play an important role in hematopoiesis in the bone marrow. In this study, mice were treated with ZA, and HSCs in bone analyzed. Altogether, the treatment differential response in the different hematopoietic progenitor and stem cells suggests that osteoclast inhibition and changes in the bone microstructure may play a role in the increased hematopoietic stem cells in the bone marrow.
MATERIALS AND METHODS
TREATMENT WITH ZOLEDRONIC ACID (ZA)
All animals were maintained in accordance with institutional animal care and use guidelines and experimental protocols approved by the Institutional Animal Care and Use Committee of the University of Michigan. Male C57BL/6J mice at 4- or 16-weeks of age were obtained from the Jackson Laboratory (Bar Harbor, ME). Intraperitoneal injections were performed twice/week with 200μg/kg of ZA (Zometa, Novartis, Stein, Switzerland) or vehicle (saline) for up to 4 weeks. At sacrifice, blood and hind limbs were collected for analyses.
FLOW CYTOMETRY ANALYSIS (FACS)
One femur and one tibia were collected and flushed with FACS buffer (PBS, 1%FBS, 2 nMEDTA). The enriched central bone marrow cells were filtered through a 100μm cell strainer and cells were enumerated and resuspended to a total of 5 million cells/500 μL. Lin cocktail (APC), Sca-1 (E13-161.7, PE), c-kit (2B8, FITC) antibodies were used to quantify the Lin-Sca1+c-Kit+ cells. To evaluate the SLAM population (CD48− CD41− CD150+ Sca1+ c-kit+) cells were incubated with the antibodies Sca-1 (E13-161.7, biotin), c-kit (2B8, APC), CD48 (HM48-1, FITC), and CD41 (MWReg30, FITC), CD150(TC15-12F12.2, PE-Biolegend). For the long-term reconstitution assay analysis, peripheral blood cells were stained with CD3, CD5, B220 (Ra3-6B2, PE). Endothelial progenitor cells (EPC) were identified as CD11b− (M1/70, APC) CD34+ (RAM34, FITC-eBioscience) and Flt1+(Avas12a1, PE-eBioscience) cells. After antibody incubation cells were washed twice with PBS and evaluated with a FACsCalibur (BD Bioscience, San Jose, CA). All other antibodies used for flow cytometry were purchased from BD Bioscience.
LONG-TERM COMPETITIVE RECONSTITUTION ASSAYS
For in vivo enumeration of HSCs, 300,000 CD45.2 bone marrow cells were isolated from ZA-treated and untreated C57BL/6 mice and were mixed with 300,000 CD45.1 cells from CD45.1 congenic mice. Cells were then intravenously injected into CD 45.2/CD45.1 recipient mice that had been lethally irradiated with 850 cGy of irradiation. Four weeks after transplantation, the frequency of the different cell populations were determined in the peripheral blood (PB) by fluorescence-activated cell sorting (FACS) using PE-CD45.1 and FITC-CD45.2 antibodies (BD Pharmingen) over a 3 month period.
HISTOLOGY AND IMMUNOHISTOCHEMISTRY
After 4 weeks of treatment, mice were sacrificed and tibiae were collected and fixed in 10% formalin. Decalcification was performed in 10% EDTA for 14 days prior to paraffin embedding. Paraffin-embedded specimens were sectioned at 5 μm and stained with hematoxylin and eosin (H&E), or tartrate-resistant acid phosphatase (TRAP) to detect osteoclasts (Acid Phosphatase, Leukocyte Kit, Sigma, St. Louis, MO) or with Von Willebrand factor (vWF) to identify vascular spaces. For microvessel density (MVD) analysis, after vWF staining, four random areas were selected. Any single or cluster of positively stained endothelial cells that was clearly separated from adjacent microvessels was considered as one countable microvessel. The average MVD was determined for each specimen. Mayer’s hematoxylin (Sigma) was used for counterstaining.
RNA ISOLATION AND QUANTITATIVE PCR ASSAY
One tibia and femur were flushed with PBS and cells were sorted into Lin−, Lin+ using magnetic cell sorting microbeads (MACS). After sorting, cells were pelleted and RNA was isolated with Tri reagent (Sigma) following the manufacturer’s protocols. One microgram of total RNA was reverse transcribed in a 20-μL reaction volume containing random hexamers using a reverse-transcription assay system (Applied Biosystems, Foster City, CA). Quantitative reverse transcription-PCR (RT-PCR) was performed using the ABI PRISM 7700 with a ready-to-use mix of primers and FAM-labeled probe assay system (Applied Biosystems). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a reference and the delta delta CT method were used to calculate the data.
SERUM TRAP5b AND CALCIUM MEASUREMENT
Serum TRAP5b activity was measured by ELISA (Immunodiagnostic Systems, Inc.) following the manufacturer’s instructions. A quantitative colorimetric assay with Calcium Reagent Set (Pointe Scientific, Inc., Canton, MI) was used to measure the total calcium levels in serum and bone marrow supernatants.
MIROFIL AND MICRO CT ANALYSES
A perfusion technique using the radiopaque silicone rubber injection agent Microfil™ followed by microCT analysis was utilized as previously described [Guldberg et al., 2008; Park et al., 2012]. Briefly, mice treated with vehicle or zoledronic acid for 4 weeks were anesthetized and perfused with lactated Ringer’s solution containing heparin, followed by 10% neutral buffered formalin, and then with a Microfil™ compound of 1.04 specific gravity mixed 4:1 with a 0.92 specific gravity diluent. Femora, tibiae, and spleens were dissected, and bones were fixed and decalcified. Samples were scanned in air at a 7 um voxel size by microcomputed tomography (eXplore Locus SP (GE Healthcare Pre-Clinical Imaging, London, ON, Canada). Regions of interest were defined for both central bone vascularity and vascular regions near the growth plate, and quantitative differences in vessel numbers and sizes were determined using the stereology package of commercially available software (MicroView v2.2, GE Healthcare Pre-Clinical Imaging, London, ON, Canada).
STATISTICAL ANALYSIS
The GraphPad Instat 3 software program (GraphPad Software, San Diego, CA) was used to analyze the differences by one-way ANOVA or Student’s t-test for independent analysis. The value P<0.05 was considered statistically significant. All assays were repeated at least twice with similar results.
RESULTS
ZA TREATMENT INCREASED BONE AREA AND OSTEOCLAST ACTIVITY IN YOUNG MICE
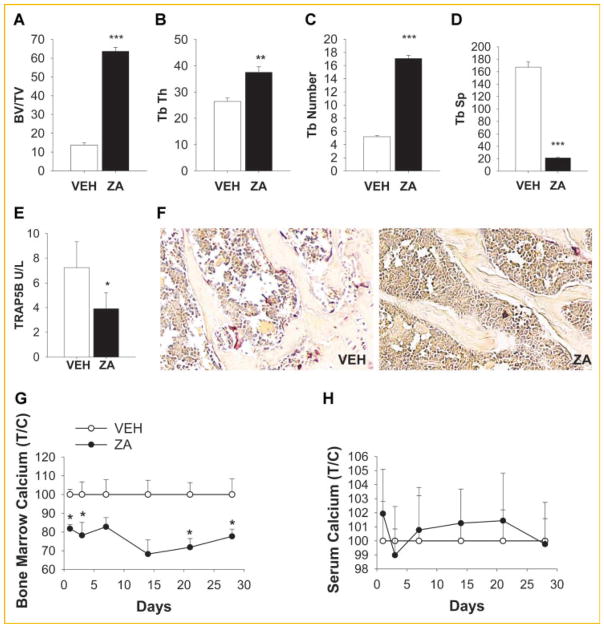
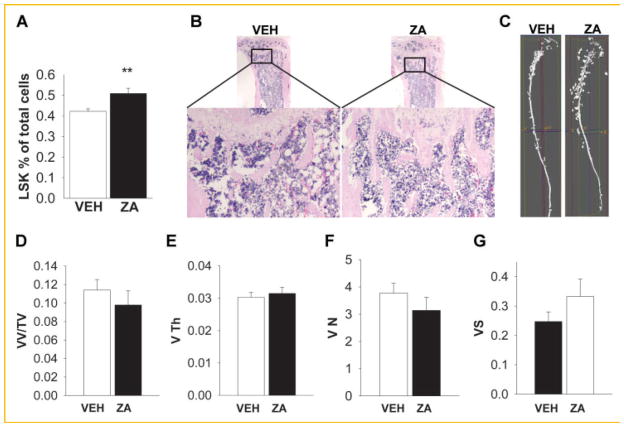
To elucidate the role of osteoclasts in the bone microenvironment, 4- week-old mice were treated with vehicle (saline) or zoledronic acid (ZA) twice a week for 4 weeks. As expected, ZA treated mice had increased bone volume, increased trabecular thickness and number, and decreased trabecular spacing (Fig. 1A–D). Serum tartrate-resistant acid phosphatase 5b (TRACP 5b) serum levels were decreased (Fig. 1E) as well as osteoclast numbers in marrow following ZA treatment (Fig. 1F). ZA treatment decreased the calcium levels in the bone marrow (Fig. 1G) but did not alter serum calcium levels (Fig. 1H) as previously reported [Li et al., 2011]. Altogether, the inhibition of osteoclasts by ZA resulted in increased bone mass.
Fig. 1.
ZA treatment effects on bone area and osteoclast activity. Four-week-old C57BL/6J male mice were treated with 200 μg/kg of ZA twice/week for 4 weeks. A–D: ZA treatment increased bone area. (A–D) Histomorphometric analysis of tibiae shows an increase of bone volume (BV/TV %), trabecular thickness (Tb Th, mm) and number(Tb number mm−1), and decreased trabecular spacing (Tb Sp,) in tibiae from ZA treated mice versus vehicle (VEH); E: blood serum TRAP5b levels were reduced with ZA treatment; F: representative TRAP staining of tibia; G–H: bone marrow calcium levels were decreased and serum calcium levels were not changed; Calcium levels were measured in the bone marrow (G) and serum (H) of mice treated with ZA or vehicle for up to 4 weeks; (T/C) Treatment/control (*P <0.05; **P <0.01; ***P <0.005 versus vehicle [VEH]). Data are presented as mean ± SEM, n = 8/gp (A–E, G–H).
HEMATOPOIETIC PROGENITOR CELLS WERE INCREASED AFTER ZA TREATMENT
To investigate the effects of osteoclast inhibition on hematopoiesis, mice were treated with ZA or vehicle for 4 weeks and the bone marrow cells were analyzed by FACS at different time points. Hematopoietic progenitor cells (Lin−Sca-1+c-kit+referred to as LSK) were significantly increased when compared to the vehicle treated mice (Fig. 2A). Since hematopoietic progenitor cells were increased the long-term reconstitution hematopoietic stem cells were also analyzed using the SLAM markers (CD48−CD41−CD150+Sca1+ c-Kit+) as previously described [Kiel et al., 2005]. As was observed with the number of LSK cells following ZA treatment, a trend of increased SLAM cells was observed with ZA treatment although it did not reach statistical significance (P = 0.056; Fig. 2B).
Fig. 2.
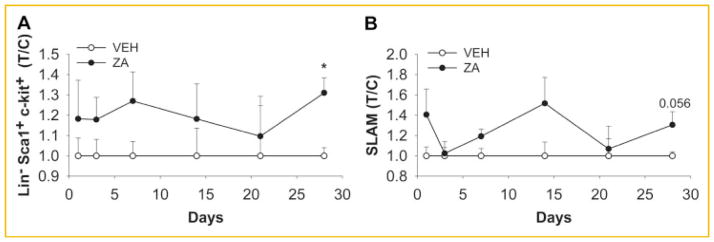
ZA treatment increased LSK population but not long-term hematopoietic stem cells. Mice were treated with 200 μg/kg of ZA twice/week for 4 weeks and bone marrow cells were analyzed by flow cytometry. A: ZA treatment increased bone marrow Lin −Sca1+ c-Kit+ hematopoietic progenitor cells after 4 weeks of treatment versus vehicle (VEH) control. B: ZA treatment did not affect the long-term hematopoietic stem populations as identified using SLAM markers (CD48−CD41−CD150+Sca1+c-Kit+; *P <0.005) T/C = treatment/control. Data are presented as mean ± SEM, n = 5/gp.
LONG-TERM RECONSTITUTION OF LYMPHOID CELLS WAS HIGHER IN ZA TREATED MICE
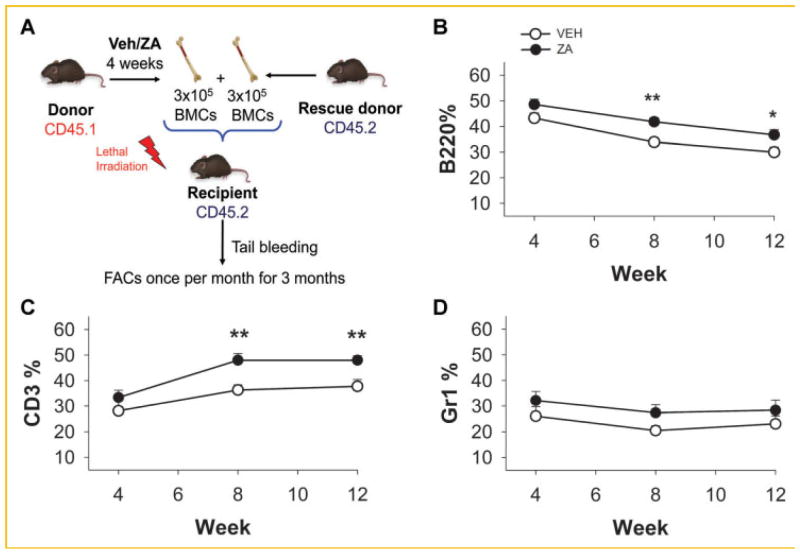
Since hematopoietic cells bearing the LSK and SLAM phenotype were increased in mice treated with ZA, their capacity for long term reconstitution was analyzed. Bone marrow cells were collected from isogenic CD45.1 mice treated with ZA or vehicle and mixed with CD45.2 donor cells at equal cell numbers (Fig. 3A). Cells were transplanted into recipient mice (CD45.2) that had received lethal irradiation and engraftment of the CD45.1 donor cells was monitored in blood over a 3 month period. Lymphoid cells were increased in mice treated with ZA with higher B and T lymphocytes (Fig. 3B,C). There were no differences in the myeloid cell populations (Fig. 3D).
Fig. 3.
Long-term HSC reconstitution was increased in ZA treated bone marrow cells. Donor CD45.1 mice were treated for 4 weeks with ZA or vehicle (VEH). Bone marrow cells were collected and mixed with rescue donor CD45.2 bone marrow cells. Mixed cells were injected intravenously in irradiated recipient mice, and blood cells were collected and analyzed by flow cytometry as indicated for CD45.1 positive and (A) B cells (B220), (B) T-cells (CD3), and (C) myeloid cells (Gr-1). Zoledronic acid treated mice had increased reconstitution of the B and T cell populations but no altered reconstitution of the myeloid Gr-1 population (**P <0.01, *P <0.05). Data are presented as mean ± SEM, n = 10.
Collectively the data show that HSCs are increased in the marrow following ZA treatment. To explore the mechanisms which could account for these data, three potential pathways which could lead to increased HSCs were explored: (1) ZA reduced hematopoietic stem cell egress or mobilization from the marrow and subsequent retention of the cells in the bone marrow, (2) ZA increased endosteal or vascular niches allowing for greater localization of HPCs/HSCs, or (3) ZA altered stem cell composition and differentiation.
HEMATOPOIETIC STEM CELL MOBILIZATION
To determine the extent to which ZA alters HSC egress or mobilization from the marrow, peripheral blood and spleens were analyzed by FACS in mice treated with ZA or vehicle. As a result of the ZA treatment, LSK numbers in the peripheral blood were not altered (Fig. 4A). No correlation was seen between LSK numbers in bone marrow and peripheral blood (data not shown). To investigate whether ZA has an effect on HSC mobilization or extramedullary hematopoiesis, the spleens of vehicle or ZA treated animals were analyzed. Spleen weight/body weight and LSK numbers in mice with ZA or vehicle were not significantly different (Fig. 4B,C). Altogether, these data suggest that increased LSKs in the bone marrow were not due to mobilization effects in mice treated with ZA.
Fig. 4.
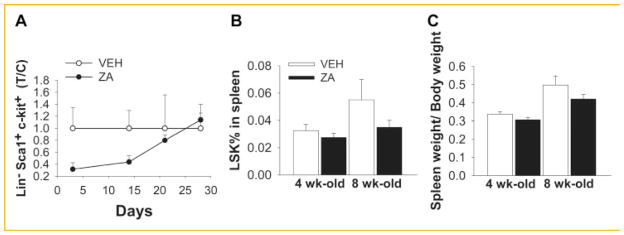
Bone marrow HSC mobilization. ZA did not alter the LSK population in the blood or spleen. Four-week-old C57BL/6J male mice were treated with 200μg/kg of ZA twice/week for 4 weeks, and peripheral blood and spleens were analyzed. A: Flow cytometry analyses of LSK population in the peripheral blood; B,C: spleen cells were collected and LSK population was analyzed by flow cytometry (B); spleen weight/body weight were analyzed and no changes were observed (C). Data are presented as mean ± SEM, n = 10/gp (A), n = 8/gp (B,C).
ENDOSTEAL AND VASCULAR NICHES
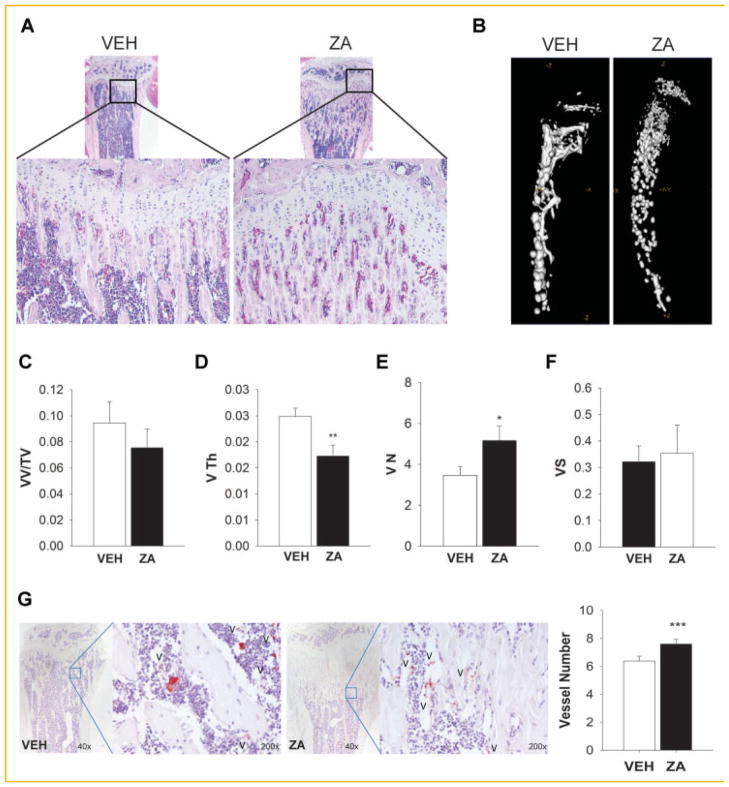
The hematopoietic niche is formed in the bone marrow by hematopoietic and non-hematopoietic cells and localized in the endosteum (area between bone marrow and bone) and sinusoids. ZA increases bone mass and thus provides an increase in niches to support HSCs. Interestingly, we observed in the bone sections of mice that augmented trabecular bone was also followed by increased small vessels number in 4 week old mice (Fig. 5A). Therefore, changes in the vasculature of bone in mice treated with ZA or vehicle for 4 weeks were examined. Radiopaque silicone rubber agent Microfil™ was perfused intravenously and micro CT analysis of vascular spaces was performed (Fig. 5B–F). Although the overall vessel volume fraction was not affected (Fig. 5C), ZA treated mice had reduced vessel thickness (Fig. 5D) and increased vessel numbers (Fig. 5E). In addition, bone sections were stained for Von Willebrand factor (vWF) and vessel numbers were quantified (Fig. 5G). Consistent with the Micro CT analyses, vessel number was increased with ZA treatment.
Fig. 5.
Effects of ZA on bone and vasculature. ZA did not alter vessel volume but increased vessel numbers in 4-week-old mice treated with ZA or vehicle (VEH) for 4 weeks. A: H&E staining of mice treated with vehicle or ZA shows an increase of vessels in the trabecular area of mice tibiae; B–F: mice were perfused with radiopaque silicone rubber microfil and tibiae were analyzed by micro CT; (B) Representative micro CT image of bones with microfil perfusion; C–F: MicroCT analysis of microfil for vessel volume/tissue volume (VV/TV%), thickness (V Th, mm), numbers (VN, mm−1), and spacing (VS, mm). ZA treatment decreased vessel thickness and increased vessel numbers; G: tibiae sections were stained for Von Willebrand factor (V) and vessel numbers were quantified (*P <0.05, **P <0.01, ***P <0.005). Data are presented as mean ± SEM, n = 10/gp.
HEMATOPOIETIC CELL COMPOSITION
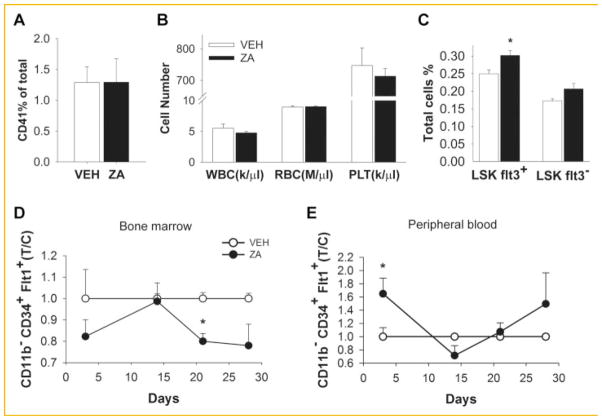
The changes seen in the hematopoietic progenitor cell populations could also be due to changes in the hematopoietic composition of the bone marrow. To assess whether ZA affected the mature hematopoietic composition, bone marrow was analyzed for CD41+ cells by FACS and no significant changes were observed (Fig. 6A). In addition, peripheral blood cell count was performed and there was no alteration in the white and red blood cells or platelets (Fig. 6B).
Fig. 6.
Effects of ZA on hematopoietic cell composition. To investigate the effects of ZA on hematopoiesis and progenitor cells, bone marrow and blood cells of 4-week-old mice were analyzed; A: no alteration in the bone marrow CD41+ mature hematopoietic composition with ZA treatment versus vehicle(VEH) control; B: blood cell count of white blood cells (WBC), red blood cells (RBC), and platelets (PLT) were not altered with ZA treatment; C: sixteen-week-old mice were treated with ZA or vehicle (VEH) for 4 weeks and bone marrow cells were collected and LSK Flt3+ and Flt3− were analyzed by flow cytometry. D–E: Endothelial progenitor cells (CD11b− CD34+ Flt3+) were analyzed over time to investigate the effects of ZA in bone marrow and mobilization to the peripheral blood; (D) EPCs in the bone marrow were significantly decreased at 3 weeks of ZA treatment but not significantly different at 4 weeks; (E) EPCs in the peripheral blood were not altered by ZA treatment (*P <0.05). Data are presented as mean ± SEM, n = 8/gp (T/C) treatment/control.
The other possibility could be that zoledronic acid induced changes in the bone could be affecting the hematopoietic stem cell differentiation into progenitor cells. Thus, we decided to further investigate the constitution of the HSC population. Stem cells are classified according to their repopulation capacity into long-term HSCs (LT-HSC) and short-term HSCs (ST-HSCs). The LT-HSCs are stem cells that present extensive life long, self-renewing potential and are capable of repopulating the whole hematopoietic system. In contrast, ST-HSCs have a lower self-renewing capacity but a rapid capability of myeloerythroid cell repopulation [Yang et al., 2005]. They can be identified according to the expression of cytokine polypeptide deformylase (fms)—like tyrosine kinase receptor 3 (Flt3): long-term-HSCs are LSK CD34−Flt3− and short-term-HSCs (LSK CD34+) can be further divided into two subsets. ST-HSCs Flt3+ for cells with great lymphoid reconstitution and ST-HSCs Flt3− for rapid myelopoiesis. Because increased LSK numbers were observed, we investigated the differentiation stage of the LSK population. Indeed LSK Flt3+ (ST-HSCs) were increased with ZA treatment (Fig. 6C) suggesting that cells with great lymphoid reconstitution are augmented by ZA induced changes in the bone microenvironment, as seen in the long term reconstitution assay (Fig. 3B,C).
To investigate whether ZA influenced bone angiogenesis, we also measured endothelial progenitor cells (EPCs) by FACS in both bone and peripheral blood (Fig. 6D,E). ZA treatment significantly decreased CD11b− CD34+ Flt1+ EPCS at 3 weeks only but at 4 weeks no difference was seen (Fig. 6D) in bone or in the peripheral blood (Fig. 6E).
GENE EXPRESSION ANALYSES
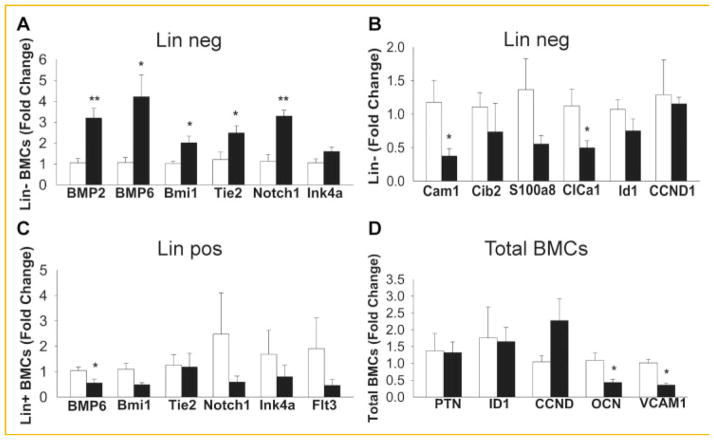
To further confirm the HSC population commitment, bone marrow cells were sorted into Lin− or Lin+ cells and gene expression of hematopoietic related genes were analyzed. Lineage negative cells had increased expression of BMP2, BMP6, Bmi1, Tie2, Notch1, but no change in Ink4a, in mice treated with ZA (Fig. 7A). BMP2 and BMP6 are proteins known to support endosteal niche maintenance, suggesting an active participation of HSCs in the endosteal niche. Bmi1, Ink4a, and Tie2 genes are indicators of self-renewal capacity [Park et al., 2003]. Bmi1 is required for HSC self-renewal since Bmi1−/− mice have normal HSCs in fetal liver but decreased HSC numbers in postnatal mice. p16Ink4a is downstream of Bmi1 and participates in the regulation of HSC proliferation [Smith et al., 2003].
Fig. 7.
Gene expression analyses of bone marrow sorted cells: lineage negative (A,B), lineage positive (C), and unsorted total bone marrow cells (D). Bone marrow cells of 4-week-old mice treated with ZA for 4 weeks were sorted into Lin− and Lin+ cells by magnetic cell sorting beads (MACS) and gene expression analyzed by qPCR. (A) Lineage negative cells had increased expression of BMP2, BMP6, BMi1, Tie2, Notch1, but no alteration of Ink4a, in mice treated with ZA versus vehicle (VEH) control. (B) Lineage negative cells were also analyzed for calcium related genes. Cam1 and ClCa1 were decreased with ZA. (C) Lineage positive cells had decreased gene expression for BMP6 but no significant changes were observed in Bmi1, Tie2, Notch1, Ink4a, and Flt3. (D) Total bone marrow cells had decrease gene expression of OCN and VCAM1 genes (*P <0.05, **p,0.005). Data are presented as mean ± SEM, n = 5/gp.
Because ZA may affect the calcium resource in bone due to the inhibition of bone resorption we also investigated calcium related genes in linage negative cells and found calmodulin 1 (Cam1) and calcium chloride channel (ClCa1) were decreased with ZA treatment (Fig. 7B).
Linage positive cells had decreased gene expression for BMP6 but no significant changes were observed in Bmi1, Tie2, Notch1, Ink4a, and Flt3 (Fig. 7C). Total bone marrow cells had decreased gene expression of osteocalcin (OCN) and vascular cell adhesion molecule 1 (VCAM1) genes (Fig. 7D). Altogether, the increased LSK population may be in part due to increased self-renewal capacity and suppression of linage commitment towards short-term hematopoietic stem cells.
ADULT MODEL VASCULATURE AND HSCS
Because the experiments were performed in 4-week-old mice that are in an active phase of growth and high bone activity, adult mice were also investigated. Four-month-old mice were treated with ZA or vehicle for 4 weeks and same analyses were performed. LSK numbers were again increased in ZA treated mice (Fig. 8A). Micro CT analyses of Microfil perfused mice presented no changes in vessel volume, thickness or number, suggesting that increased LSK numbers in 4-month-old mice were not due to increased vascular niches (Fig. 8B–G).
Fig. 8.
Effects of ZA on bone and vasculature in 16-week-old mice. ZA did not alter vessel volume (VV/TV), thickness (V Th), or number(VN) in adult mice, but still increased LSK numbers. Sixteen-week-old mice were treated with ZA or vehicle (VEH) for 4 weeks. A: Bone marrow cells were collected and LSK were analyzed by flow cytometry. B: H&E staining of mice treated with vehicle or ZA shows similar vessels in the trabecular area of tibiae. C: Representative MicroCT images of bones with microfil perfusion. D–G: MicroCT analyses of microfil for vessel volume/tissue volume (VV/TV%), thickness (V Th, mm), number (VN, mm−1), and spacing (VS, mm; **P <0.005). Data are presented as mean ± SEM, n = 8/gp.
DISCUSSION
In this study, treatment with ZA in both young and adult mice significantly increased the hematopoietic progenitor population (LSK) in the bone marrow. The augmented LSK population was confirmed functionally by long term reconstitution assays showing increased repopulation of B and T lymphocytes in mice that received bone marrow stromal cells from donor mice treated with ZA. One interesting finding was that reconstitution of Gr-1+ population that accounts for myeloid cells was not different. A recent study demonstrated that TGF-β1 can regulate the hematopoietic stem cell long–term differentiation. High levels of TGF-β1 resulted in stimulation of myeloid-biased HSCs and inhibition of lymphoid-biased HSCs [Challen et al., 2010]. ZA is known to inhibit bone resorption and consequently reduce the release of TGF-β1 from resorbed bone[Wu et al., 2010]. Accordingly, with ZA treatment an increased reconstitution of lymphocyte B and T cells were observed but not the myeloid population suggesting that low TGF-β1 could be modulating the reconstitution of these cells in the bone marrow. Moreover, ZA treatment increased the LSK Flt3+ population that are known to be short term lymphocytic reconstituting cells, confirming an effect of ZA in hematopoietic reconstitution.
Since LSK cells were increased in ZA treated mice, 3 potential hypotheses were investigated; (1) mobilization of LSK in the bone marrow, (2) increased bone marrow niches supporting increased numbers of LSKs, and (3) altered cell composition and stem cell differentiation.
Mobilization depends on detachment of HSCs from their niches concomitantly with cellular migration and egress from bone marrow to the circulation. Mobilization strategies have been used clinically to collect a large number of hematopoietic stem cells from the blood, a less invasive procedure than bone marrow aspiration for transplantation. Regulation of the bone microenvironment and the niches present in the marrow are important to preserve HSCs and therapies that interfere in this balance can affect the stem cell pool. Based on our findings, mobilization of LSKs in ZA treated mice was not altered since the LSK population was unchanged in the peripheral blood as well as the spleens. No difference was seen in white and red blood count or in the platelet numbers in peripheral blood. Studies demonstrated that bone remodeling is altered when HSC mobilization is induced by granulocyte-colony stimulating factor (G-CSF) treatment with decreased bone volume [Lee et al., 1991; Takahashi et al., 1996]. Therefore, bone activity is closely associated with HSC commitment for mobilization and differentiation with egress of HSCs from the bone marrow. Interestingly ZA inhibits osteoclast function and subsequently results in increased bone volume, an opposite effect seen in G-CSF therapies.
Since ZA treatment did not stimulate the egress of LSKs from the bone marrow to the circulation two other questions were addressed. First was whether ZA promotion of LSK increase is due to hematopoietic niche alteration and secondly, whether ZA regulates the factors necessary for the retention of HSCs with modulation of cell commitment. ZA is a bisphosphonate clinically utilized for the treatment of bone associated diseases such as osteosporosis and skeletal malignancies due to its inhibitory actions in osteoclast mediated bone resorption. ZA actions in bone were confirmed by histomorphometric analyses of bone sections demonstrating a significant increase bone mass and decrease in osteoclast activity. Hematopoietic niches maintain self-renewing and dormant HSCs in the bone marrow and although still controversial, HSCs are believed to reside in association with bone locals termed endosteal niches and surrounding sinusoids present in the marrow, known as vascular niches. It was also proposed that the distinct niches have different functionalities: the endosteal niche homes and maintains long-term HSCs and the vascular niche is involved in the short term HSC maintenance, promoting the differentiation and circulation of HSCs [Grassinger et al., 2010]. However, to fully differentiate both niches is difficult due to the close association of the vasculature especially in trabecular bone rich areas, locations where HSCs preferentially lodge [Ellis et al., 2011]. An increase in the osteoblastic niche could account for the augmented LSK population, especially for the long-term LSKs that were modestly increased after ZA treatment using the SLAM markers.
Bisphosphonates are being studied for their activities not only in the bone microenvironment but also putative direct effects in tumor cell proliferation, adhesion, invasion, apoptosis, and angiogenesis [Rogers and Holen, 2011]. The anti-angiogenic effects of ZA have been demonstrated by its actions as a potent inhibitor of endothelial cell proliferation, adhesion migration and angiogenesis [Wood et al., 2002; Metcalf et al., 2011]. However, ZA effects in bone vasculature are still poorly defined. Bone vascular biology is an understudied area and the interdependency of vascular physiology and osteogenesis needs to be more thoroughly investigated. It is known that angiogenesis and bone formation are intrinsically linked during bone development [Wang et al., 2007] and repair [Wan et al., 2008]. Osteoclasts actively participate in this process, excavating the marrow cavity, while sinusoids establish this unique type of bone microcirculation [Bianco, 2011]. Although biological evidence is observed in the bone coupling with endothelial cells [Parfitt, 2000], the formation and modulation of the vasculature linked with bone modeling and the effects of stimulators (hormones, drugs and mechanical loading) that affect its microstructure are relatively unexplored. Studies in mechanical loading, demonstrated that bone angiogenesis was accompanied with bone gain in running rats [Yao et al., 2004] and VEGF was the mechanism driving both effects. Recently, an interesting study demonstrated that intermittent parathyroid hormone (PTH) induced vascular endothelial growth factor (VEGF A) production that mediated PTH anabolic response [Prisby et al., 2011]. PTH treated mice had fewer blood vessel numbers and relocated existing blood vessels near to new bone formation sites. Interestingly, ZA treatment decreased the circulating levels of VEGF in cancer patients with bone metastasis [Santini et al., 2003] but their effects on the bone vasculature were not determined.
In this study, we observed the expected increase in bone mass, but we also investigated the changes that occur in the vasculature of mice treated with ZA. To this end, perfusion of a radiopaque microfil was performed to visualize and quantify changes in the vasculature volume and vessel numbers. Interestingly, young mice (4 week old) treated with ZA had no alteration in overall vessel volume but had increased vessel numbers that were smaller and accumulated between the augmented trabecular areas in the metaphysis. This finding lead us to further investigate if the changes in the bone niche (both endosteal and vascular) microstructures contributed to the increased LSK numbers. Because the experiments were performed in young (4week old) mice where bone is highly active, we also performed experiments in adult (4 month old) mice. Similar to the young mice, LSK cells and bone mass were significantly increased after 4 week treatment with ZA but surprisingly vessel numbers and volumes were not changed in the adult mice. The increase in bone vessel numbers in the young model but not in the adult mice suggests that ZA may act differently depending on the age and bone activity. Moreover, the fact that ZA is a known antiangiogenic factor, but did not reduce vessel number or volume in bone raises interesting questions regarding the mechanisms by which bone angiogenesis occurs and how important their interdependency is with bone modulation and activity. Further studies are necessary to fully elucidate bone angiogenesis and its contribution to the bone microenvironment. Collectively, the findings suggest that ZA indirectly supports the osteoblastic niche and that the vasculature niche may not be a key player in the augmented LSK population in bone.
To account for an increase of LSK in the marrow, the present study sought to determine whether ZA would affect other cell types in the bone marrow. Analysis of hematopoietic CD41+cells revealed similar levels between ZA and VEH. Moreover, investigation of different LSK populations was performed to determine whether ZA inhibited cell commitment that could account for the increased population in the bone marrow. Indeed, short-term lymphocytic reconstituting LSK Flt3+ cells were increased with ZA treatment. Lymperi et al. [2011] demonstrated that inhibition of osteoclastic resorption with alendronate treatment resulted in expansion of hematopoietic progenitor cells (LSK) but with a decrease in the absolute number of LSK Flt3− HSCs and long-term culture-initiating cells in the bone marrow. However, alendronate is a second generation bisphosphonate with a lower efficacy of osteoclast inhibition than ZA.
Gene expression of Lin− sorted cells demonstrated that Bmi1 and Tie2, related with HSC self-renewal [Arai et al., 2004; Rizo et al., 2008], were both increased, suggesting that hematopoietic progenitor cells are acquiring self-renewal capacity. Interestingly, BMP2 and BMP6 were both augmented with ZA treatment in Lin negative cells. Jung et al. [2008] demonstrated that hematopoietic stem cells participate in bone formation and niche activity with secretion of BMP2 and BMP6. Later, a role of erythropoietin for BMP2 and BMP6 production was reported [Shiozawa et al., 2010]. Indeed, the endosteal niche is expanded with ZA treatment and hematopoietic progenitor cell could be actively participating in the bone formation. It is possible that as osteoclasts are compromised with ZA treatment their coupling factors to stimulate osteoblasts (e.g., ephrins)are compromised and HPCs are serving to compensate. Notch 1, a receptor for ligand Jagged 1 expressed by osteoblasts, was also increased and is involved in the expansion of the HSC pool in vivo [Calvi et al., 2003].
Altogether, accordingly with increased endosteal niches and increased LSKs, our data suggest that ZA indirectly supports the osteoblastic niche LSK expansion that occurs independent of the vascular niche. Moreover, ZA has a differential response in the vasculature modulation in young and adult mice, suggesting that age and bone activity may be significant factors defining the vasculature modulation in bone.
Acknowledgments
Grant sponsor: National Cancer Institute; Grant number: P01CA093900; Grant sponsor: National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases; Grant number: DK53904.
We thank Chris Strayhorn for histologic assistance, the Flow Cytometry core and the Comprehensive cancer center at University of Michigan for the flow cytometry analyses, and Sean J. Morrison, PhD (Formerly of the University of Michigan) for assistance in the long-term reconstitution assay.
Footnotes
The authors declare no conflicts of interest.
References
- Adams GB, Chabner KT, Alley IR, Olson DP, Szczepiorkowski ZM, Poznansky MC, Kos CH, Pollak MR, Brown EM, Scadden DT. Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature. 2006;439:599–603. doi: 10.1038/nature04247. [DOI] [PubMed] [Google Scholar]
- Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, Ito K, Koh GY, Suda T. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118:149–161. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Bianco P. Bone and the hematopoietic niche: A tale of two stem cells. Blood. 2011;117:5281–5288. doi: 10.1182/blood-2011-01-315069. [DOI] [PubMed] [Google Scholar]
- Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, Milner LA, Kronenberg HM, Scadden DT. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- Challen GA, Boles NC, Chambers SM, Goodell MA. Distinct hematopoietic stem cell subtypes are differentially regulated by TGF-beta1. Cell Stem Cell. 2010;6:265–278. doi: 10.1016/j.stem.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho KA, Joo SY, Han HS, Ryu KH, Woo SY. Osteoclast activation by receptor activator of NF-kappaB ligand enhances the mobilization of hematopoietic progenitor cells from the bone marrow in acute injury. Int J Mol Med. 2010;26:557–563. doi: 10.3892/ijmm_00000499. [DOI] [PubMed] [Google Scholar]
- Ellis SL, Grassinger J, Jones A, Borg J, Camenisch T, Haylock D, Bertoncello I, Nilsson SK. The relationship between bone, hemopoietic stem cells, and vasculature. Blood. 2011;118:1516–1524. doi: 10.1182/blood-2010-08-303800. [DOI] [PubMed] [Google Scholar]
- Grassinger J, Haylock DN, Williams B, Olsen GH, Nilsson SK. Phenotypically identical hemopoietic stem cells isolated from different regions of bone marrow have different biologic potential. Blood. 2010;116:3185–3196. doi: 10.1182/blood-2009-12-260703. [DOI] [PubMed] [Google Scholar]
- Guldberg RE, Duvall CL, Peister A, Oest ME, Lin AS, Palmer AW, Levenston ME. 3D imaging of tissue integration with porous biomaterials. Biomaterials. 2008;29:3757–3761. doi: 10.1016/j.biomaterials.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung Y, Song J, Shiozawa Y, Wang J, Wang Z, Williams B, Havens A, Schneider A, Ge C, Franceschi RT, McCauley LK, Krebsbach PH, Taichman RS. Hematopoietic stem cells regulate mesenchymal stromal cell induction into osteoblasts thereby participating in the formation of the stem cell niche. Stem Cells. 2008;26:2042–2051. doi: 10.1634/stemcells.2008-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- Kollet O, Dar A, Shivtiel S, Kalinkovich A, Lapid K, Sztainberg Y, Tesio M, Samstein RM, Goichberg P, Spiegel A, Elson A, Lapidot T. Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat Med. 2006;12:657–664. doi: 10.1038/nm1417. [DOI] [PubMed] [Google Scholar]
- Lee MY, Fukunaga R, Lee TJ, Lottsfeldt JL, Nagata S. Bone modulation in sustained hematopoietic stimulation in mice. Blood. 1991;77:2135–2141. [PubMed] [Google Scholar]
- Li X, Liao J, Park SI, Koh AJ, Sadler WD, Pienta KJ, Rosol TJ, McCauley LK. Drugs which inhibit osteoclast function suppress tumor growth through calcium reduction in bone. Bone. 2011;48:1354–1361. doi: 10.1016/j.bone.2011.03.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lymperi S, Ersek A, Ferraro F, Dazzi F, Horwood NJ. Inhibition of osteoclast function reduces hematopoietic stem cell numbers in vivo. Blood. 2011;117:1540–1549. doi: 10.1182/blood-2010-05-282855. [DOI] [PubMed] [Google Scholar]
- Metcalf S, Pandha HS, Morgan R. Antiangiogenic effects of zoledronate on cancer neovasculature. Future Oncol. 2011;7:1325–1333. doi: 10.2217/fon.11.113. [DOI] [PubMed] [Google Scholar]
- Miyamoto K, Yoshida S, Kawasumi M, Hashimoto K, Kimura T, Sato Y, Kobayashi T, Miyauchi Y, Hoshi H, Iwasaki R, Miyamoto H, Hao W, Morioka H, Chiba K, Kobayashi T, Yasuda H, Penninger JM, Toyama Y, Suda T, Miyamoto T. Osteoclasts are dispensable for hematopoietic stem cell maintenance and mobilization. J Exp Med. 2011;208:2175–2181. doi: 10.1084/jem.20101890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parfitt AM. The mechanism of coupling: A role for the vasculature. Bone. 2000;26:319–323. doi: 10.1016/S8756-3282(00)80937-0. [DOI] [PubMed] [Google Scholar]
- Park IK, Qian D, Kiel M, Becker MW, Pihalja M, Weissman IL, Morrison SJ, Clarke MF. Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature. 2003;423:302–305. doi: 10.1038/nature01587. [DOI] [PubMed] [Google Scholar]
- Park SI, Liao J, Berry JE, Li X, Koh AJ, Michalski ME, Eber MR, Soki FN, Sadler D, Sud S, Tisdelle S, Daignault SD, Nemeth JA, Snyder LA, Wronski TJ, Pienta KJ, McCauley LK. Cyclophosphamide creates a receptive microenvironment for prostate cancer skeletal metastasis. Cancer Res. 2012;72:2522–2532. doi: 10.1158/0008-5472.CAN-11-2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prisby R, Guignandon A, Vanden Bossche A, Mac-Way F, Linossier MT, Thomas M, Laroche N, Malaval L, Langer M, Peter ZA, Peyrin F, Vico L, Lafage-Proust MH. Intermittent PTH(1–84) is osteoanabolic but not osteoangiogenic and relocates bone marrow blood vessels closer to bone-forming sites. J Bone Miner Res. 2011;26:2583–2596. doi: 10.1002/jbmr.459. [DOI] [PubMed] [Google Scholar]
- Rizo A, Dontje B, Vellenga E, de HG, Schuringa JJ. Long-term maintenance of human hematopoietic stem/progenitor cells by expression of BMI1. Blood. 2008;111:2621–2630. doi: 10.1182/blood-2007-08-106666. [DOI] [PubMed] [Google Scholar]
- Rodan GA, Fleisch HA. Bisphosphonates: Mechanisms of action. J Clin Invest. 1996;97:2692–2696. doi: 10.1172/JCI118722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers TL, Holen I. Tumour macrophages as potential targets of bisphosphonates. J Transl Med. 2011;9:177. doi: 10.1186/1479-5876-9-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini D, Vincenzi B, Dicuonzo G, Avvisati G, Massacesi C, Battistoni F, Gavasci M, Rocci L, Tirindelli MC, Altomare V, Tocchini M, Bonsignori M, Tonini G. Zoledronic acid induces significant and long-lasting modifications of circulating angiogenic factors in cancer patients. Clin Cancer Res. 2003;9:2893–2897. [PubMed] [Google Scholar]
- Shiozawa Y, Jung Y, Ziegler AM, Pedersen EA, Wang J, Wang Z, Song J, Wang J, Lee CH, Sud S, Pienta KJ, Krebsbach PH, Taichman RS. Erythropoietin couples hematopoiesis with bone formation. PLoS ONE. 2010;5:e10853. doi: 10.1371/journal.pone.0010853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Chanda SK, Lingbeek M, Ross DT, Botstein D, van LM, Cleary ML. Bmi-1 regulation of INK4A-ARF is a downstream requirement for transformation of hematopoietic progenitors by E2a-Pbx1. Mol Cell. 2003;12:393–400. doi: 10.1016/s1097-2765(03)00277-6. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Wada T, Mori M, Kokai Y, Ishii S. Overexpression of the granulocyte colony-stimulating factor gene leads to osteoporosis in mice. Lab Invest. 1996;74:827–834. [PubMed] [Google Scholar]
- Visnjic D, Kalajzic Z, Rowe DW, Katavic V, Lorenzo J, Aguila HL. Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood. 2004;103:3258–3264. doi: 10.1182/blood-2003-11-4011. [DOI] [PubMed] [Google Scholar]
- Wan C, Gilbert SR, Wang Y, Cao X, Shen X, Ramaswamy G, Jacobsen KA, Alaql ZS, Eberhardt AW, Gerstenfeld LC, Einhorn TA, Deng L, Clemens TL. Activation of the hypoxia-inducible factor-1alpha pathway accelerates bone regeneration. Proc Natl Acad Sci USA. 2008;105:686–691. doi: 10.1073/pnas.0708474105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wan C, Deng L, Liu X, Cao X, Gilbert SR, Bouxsein ML, Faugere MC, Guldberg RE, Gerstenfeld LC, Haase VH, Johnson RS, Schipani E, Clemens TL. The hypoxia-inducible factor alpha pathway couples angiogenesis to osteogenesis during skeletal development. J Clin Invest. 2007;117:1616–1626. doi: 10.1172/JCI31581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter MC, Coleman RE. Bisphosphonates in breast cancer: Teaching an old dog new tricks. Curr Opin Oncol. 2009;21:499–506. doi: 10.1097/CCO.0b013e328331c794. [DOI] [PubMed] [Google Scholar]
- Wood J, Bonjean K, Ruetz S, Bellahcene A, Devy L, Foidart JM, Castronovo V, Green JR. Novel antiangiogenic effects of the bisphosphonate compound zoledronic acid. J Pharmacol Exp Ther. 2002;302:1055–1061. doi: 10.1124/jpet.102.035295. [DOI] [PubMed] [Google Scholar]
- Wu X, Pang L, Lei W, Lu W, Li J, Li Z, Frassica FJ, Chen X, Wan M, Cao X. Inhibition of Sca-1-positive skeletal stem cell recruitment by alendronate blunts the anabolic effects of parathyroid hormone on bone remodeling. Cell Stem Cell. 2010;7:571–580. doi: 10.1016/j.stem.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Bryder D, Adolfsson J, Nygren J, Mansson R, Sigvardsson M, Jacobsen SE. Identification of Lin(−)Sca1(+)kit(+)CD34(+)Flt3-short-term hematopoietic stem cells capable of rapidly reconstituting and rescuing myeloablated transplant recipients. Blood. 2005;105:2717–2723. doi: 10.1182/blood-2004-06-2159. [DOI] [PubMed] [Google Scholar]
- Yao Z, Lafage-Proust MH, Plouet J, Bloomfield S, Alexandre C, Vico L. Increase of both angiogenesis and bone mass in response to exercise depends on VEGF. J Bone Miner Res. 2004;19:1471–1480. doi: 10.1359/JBMR.040517. [DOI] [PubMed] [Google Scholar]
- Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, Ross J, Haug J, Johnson T, Feng JQ, Harris S, Wiedemann LM, Mishina Y, Li L. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]