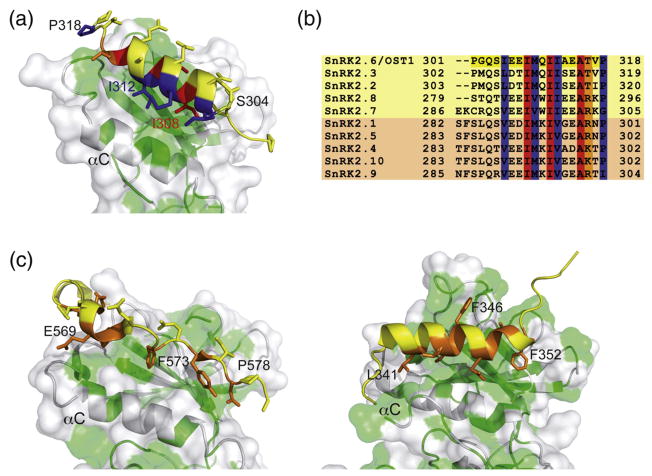
Fig. 2.
Interactions between DI and the OST1 catalytic kinase domain and related structures. (a) A section of the OST1 structure showing the residues involved in the relevant interactions between DI and the kinase domain. A cartoon representation of the kinase N-lobe is displayed together with a semitransparent surface. Hydrophobic residues of the kinase domain are highlighted in green. Residues buried in the interaction with the kinase domain are colored according to their conservation degree among the members of the family [see (b)]. (b) A comparison of the DI domain amino acids in A. thaliana SnRK2s. The kinases boxed in yellow or light orange belong to the ABA-dependent or ABA-independent regulation subgroup, respectively. (c) An equivalent section to the OST1 structure showed in (a) of the atypical protein kinase C (PDB ID: 1ZRZ) (left) and the ERK2 protein kinase (PDB ID: 1ERK) (right). Residues buried in the interaction with the kinase domain are colored orange.