ABSTRACT
Direct carotid-cavernous fistula is an abnormal arteriovenous communication between the carotid artery and the cavernous sinus occurring spontaneously or following head trauma. The aim of this paper is to report our experience and a review of the literature regarding the curative effect of endovascular treatment for patients with post-traumatic direct CCF. We present five patients with direct post-traumatic CCF in whom endovascular treatment was applied and the outcomes of the endovascular treatment. Direct post-traumatic CCF may be completely occluded without technique-related complications using detachable balloons, coils as embolic material or by using covered stents placed in the parent vessel to exclude the fistula from circulation. Postembolization angiographies revealed that the fistula was successfully obliterated. Few days after the procedure the ophthalmic symptoms were much reduced, and completely resolved soon after.
In conclusion endovascular treatment represents an effective method for complete occlusion the direct CCF no matter of the technique chosen.
Keywords: carotid-cavernous fistula, endovascular, occlusion, embolization
BACKGROUND
A carotid-cavernous fistula (CCF) is an abnormal arteriovenous communication between the carotid artery and the cavernous sinus. The fistula may occur spontaneously (25% of all cases) but usually occurs following head trauma (75%) - domestic violence, falls from height, car accident and may also be iatrogenic (0,1%) - as complications of rhinoplasty, transsphenoidal surgery, embolization of cavernous sinus meningioma, and rhinocerebral mucormycosis (1). Spontaneous (nontraumatic) direct CCFs develop in patients with intracavernous aneurysms, and in individuals who have connective tissue diseases that cause vascular wall fragility, the vascular type of Ehlers-Danlos and fibromuscular dysplasia (2).
According to the Barrow classification, there are four angiographically identifiable types of CCF (types A-D) (1,3). The type A fistula is a direct, high flow fistula between the cavernous part of internal carotid artery and the cavernous sinus. It occurs most frequently following head trauma and it may form from a traumatic tear in the wall of the cavernous internal carotid artery or following rupture of an aneurysm, high pressure arterial blood gaining rapid access to the venous system and leads to venous hypertension.
Type B-D are indirect fistulas and occur between meningeal branches of the external or internal carotid artery and the cavernous sinus, being low-flow fistulas. The etiology of indirect fistulas is not so clear, but they have been associated with pregnancy, sinusitis, age, and trauma. Because the pressure is not as high as in direct CCF symptoms are usually mild and may include dilated conjunctival and episcleral vessels and mild proptosis while the onset of symptoms in patients with direct CCF is usually abrupt and may be very dramatic. Patients usually present with neuro-ophthalmologic symptoms including venous congestion of the eyelids, conjunctiva and episcleral vessels with lid swelling, cranial nerve palsies, visual loss, proptosis, elevated intraocular pressure, optic disc edema, and dilated and tortuous retinal vessels. A triad of clinical findings is specific for this type of fistulas: exophthalmos, orbital bruit and dilated conjunctival vessels (1).
For the diagnosis of direct CCF digital subtraction cerebral angiography represents the gold standard although there are other techniques that may be helpful such as cerebral CT scan, cerebral MRI and also Doppler ultra-sound, angiography being used to confirm CT or MRI findings prior to treatment. Sometimes CT findings may be sufficient for diagnosis showing enlargement of cavernous sinus and the ophthalmic vein.
Magnetic resonance imaging techniques are superior in evaluating venous distension and the increased flow to cavernous sinus and can also demonstrate engorgement of the superior ophthalmic vein, extraocular muscle enlargement, and dilation of the affected cavernous sinus (4). In extracranial carotid Doppler examination, hemodynamic parameters of the feeding artery show an abnormally increased flow velocities and volume and decreased resistivity indices. In transcranial carotid Doppler, at orbital examination, one could detect a continuous bruit, which means absorption of blood flow from artery to venous sinus, without keeping any shape of velocimetric complexes.
CCFs often are misdiagnosed as thyroid ophthalmopathy, conjunctivitis or even orbital cellulitis.
There are several modalities of treating direct CCF: surgical treatment with ligature of the carotid artery, neurosurgical treatment, and endovascular treatment performed in 99% of the cases.
Complete occlusion of the fistula may be obtained by endovascular approach either with detachable balloons, coils and other solid or liquid agents used as embolic material or by using covered stents placed in the parent vessel to exclude the fistula from circulation. It is to mention that cerebral angiography may determine spontaneous closure of the CCF due to irritation of the vessels and compression of the carotid artery (4).
Using a microcatheter a silicone balloon is guided to the fistula and gradually inflated as it approaches the cavernous sinus. Once there and the fistula occluded the balloon is detached. The balloon may be filled with hydroxyethylmethacrylate, which polymerizes within an hour at body temperature and makes the balloon less likely to deflate or migrate than iodinated contrast alone (5). There are some cases when detachable balloons cannot be used, especially if preservation of the parent artery is desired: a small orifice that does not allow the balloon passage, a small cavernous sinus that causes the balloon to be herniated into the parent internal carotid artery, sharp osseous fragments within the sinus that can cause rupture balloon on inflation, a markedly enlarged cavernous sinus that cannot be completely filled by even multiple balloons, which leaves empty spaces that prevent complete occlusion and cure, and transection of the cavernous internal carotid artery. In addition, the use of detachable balloons may also be limited by their availability (6-8).
We have to remind another option for the occlusion of direct CCF using coils which may be done transarterial or transvenous but this technique has a risk of parent artery occlusion due to herniation of a coil loop into the parent artery (5,7,9-12). Because direct CCFs are high flow fistulas the use of solid (polyvinyl alcohol particles) or liquid agents (cyanoacrylate monomers, ethylene vinyl alcohol, absolute ethanol) as embolic material have a great risk for distal migration (13), sometimes determining cerebral venous infarction from reflux of glue into the cortical venous structures [14]. In some cases it may be used a combination between detachable coils and embolic materials described above.
The most recently method for occlusion of direct CCF is the use of graft-stents used to obliterate fistulas directly or to provide support for coils (7). Because of their stiffness, they must be gently advanced otherwise complications such as dissection along the edge of a stent causing a pseudoaneurysm, which may lead to hemorrhage or thromboembolism; or deformation and migration of a stent may appear causing intracranial artery occlusion and massive cerebral infarction (7,15). Another problem to take in consideration is an endoleak between a graft-stent and the parent artery. Gomez et al. used an additional graft-stent or detachable coils to treat residual fistula filling, and Archondakis et al. reported that postdilation using a coronary balloon of larger diameter is often needed to exclude an endoleak completely after graft-stent placement for traumatic CCF (8,16,17). Another important problem is the artery patency on short and long-term when using stent-grafts. Recent evidences suggest a positive long-term outcome (7,8,18). ❑
CASE REPORTS
Case 1
A 66 years old female presented one month after a trauma caused by aggression with progressive unilateral visual loss, exophthalmos, proptosis and lid swelling.
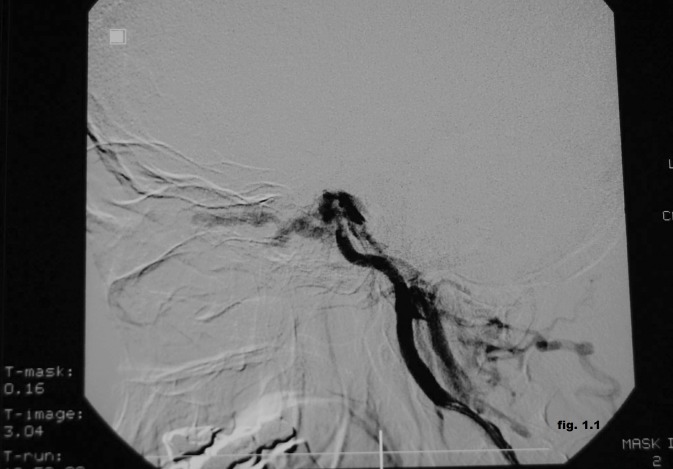
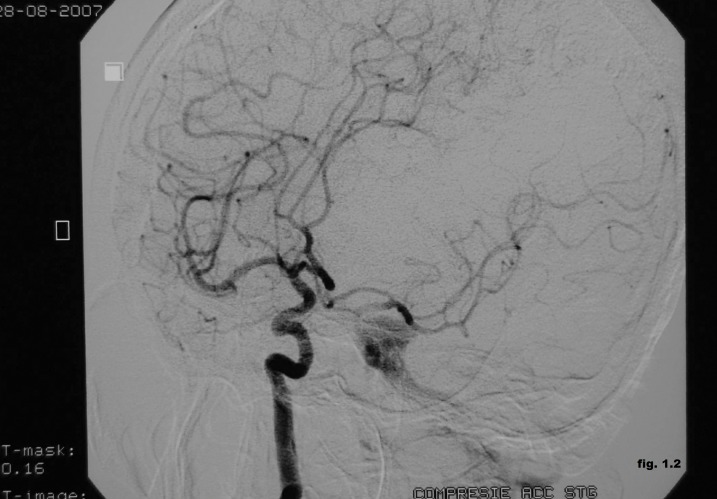
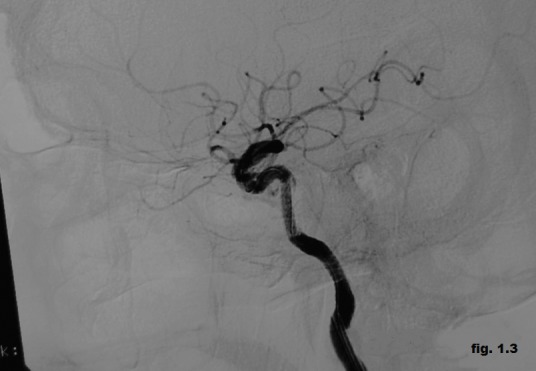
The cerebral magnetic resonance imaging showed enlargement of cavernous sinus and distension the ophthalmic vein with increased flow to cavernous sinus and the angiography proved the presence of the left post-traumatic direct CCF (Figure 1.1). It was performed the embolization of the CCF using the stent-graft technique (Figure 1.2). It was used percutaneuos access via the right femoral artery with insertion of a 6F sheath. A 6F guiding catheter (Envoy) was positioned in the left ICA. A 0.014-inch microwire was navigated into the M1 segment of the left middle cerebral artery. A 3x16 mm-sized graft-stent (GraftMaster, Jostent) was then advanced and positioned across the horizontal part of the intracavernous segment of the left internal carotid artery at the site of carotid-cavernous effraction followed by postdilatation with a 4x12 mm balloon. During the intervention it was administrated 5000 U.I of heparin. The complete obliteration of the CCF was obtained with normal angiographic aspect of the intracerebral vessels, without neurologic complication. Few days after the procedure the ophthalmic symptoms were much reduced, and completely resolved soon after. The patient was discharged seven days after the procedure on 100 mg aspirin and 75 mg clopidogrel daily. Follow-up angiography (Figure 1.3) demonstrated complete occlusion of the post-traumatic CCF without complications such as in-stent stenosis or an endoleak.
Figure 1.1. Cerebral angiography showing the presence of the left post-traumatic direct CCF.
Figure 1.2. Embolization of the CCF using the stent-graft technique.
Figure 1.3. Follow-up angiography (complete occlusion of the fistula).
Case 2
A 31 years old female admitted to our clinic for loss of visual sharpness, bilateral exophthalmia (L>R), important chemosis, limitation of the ocular motility and ocular pain two months after a fall from height.
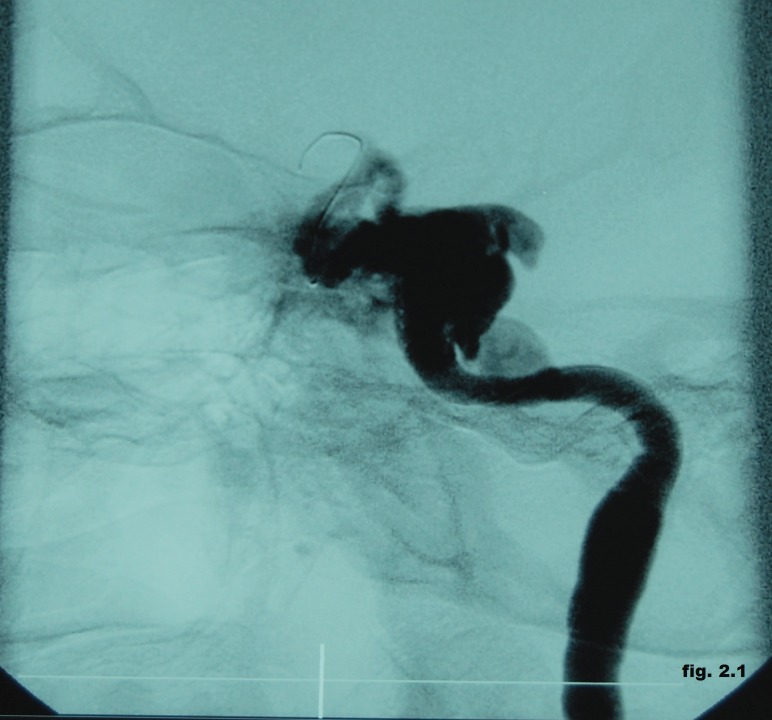
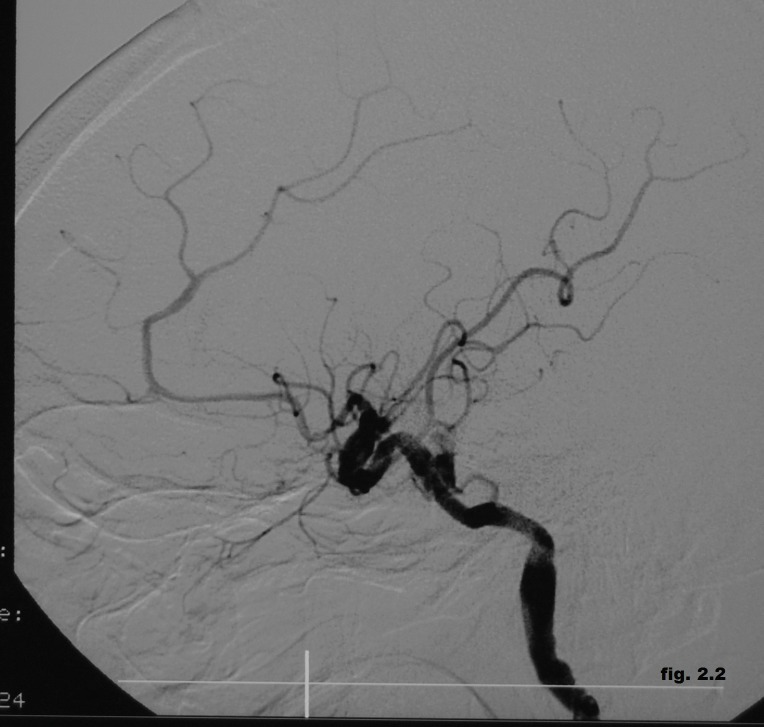
The angiography confirmed the presence of a direct CCF with ophthalmic veins distension and inverted flow with the area of arterio-venous communication situated in the petrous ascending segment of the left internal carotid artery (Figure 2.1). We choose the endovascular treatment by angioplasty with a 4x12 mm stent-graft positioned in the left internal carotid artery across the fistula but the control angiogram showed the incomplete obliteration of the fistula. Therefore, we decided to use another 3x16 mm stent-graft, proximally to the first one, followed by repeated post-dilatations with a 3x20 mm balloon (Figure 2.2).
Figure 2.1. Direct CCF with ophthalmic veins distension and inverted flow with the area of arterio-venous communication situated in the petrous ascending segment of the left internal carotid artery.
Figure 2.2. Endovascular treatment by angioplasty with two stent-grafts positioned in the left internal carotid artery across the fistula.
Final angiograms confirmed normal patency of the internal carotid artery and total exclusion of the fistula, showing the lack of filling of the venous system and normal intra-cerebral aspect.
After the procedure, there were no new neurological findings. Furthermore, the ocular pain was much reduced.
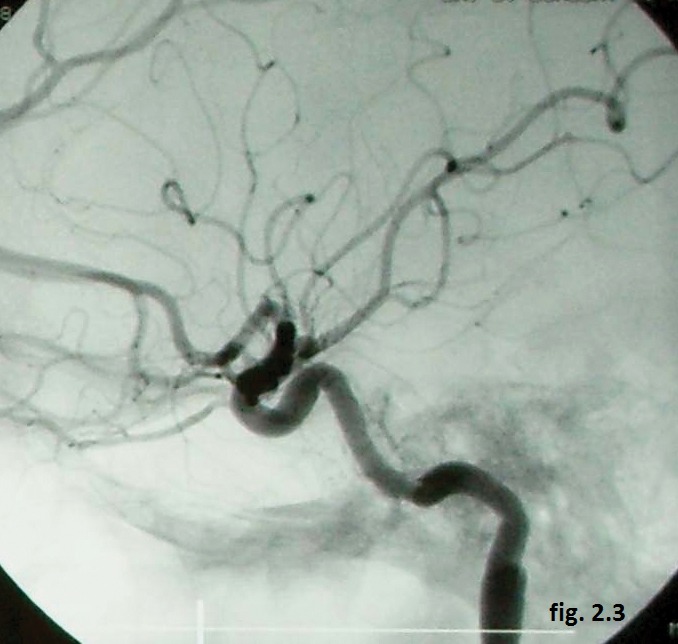
Follow up angiography (Figure 2.3) six months later demonstrated stable occlusion of the CCF and good internal cerebral artery patency and the neurologic and clinical exams performed one year later were normal.
Figure 2.3. Follow up angiography (stable occlusion of the CCF).
Case 3
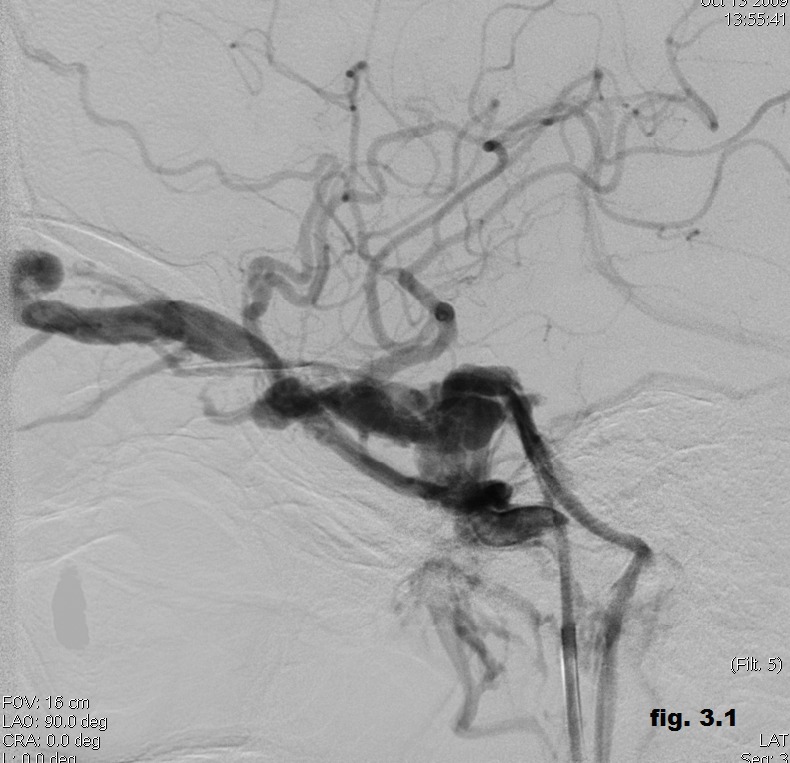
A 22 years old male who presented three weeks after a motorcycle accident with left exophthalmos, ptosis, conjunctival injection, loss of visual sharpness, limitation of the ocular motility and ocular pain. Magnetic resonance imaging of the orbits confirmed the presence of a markedly ectatic left superior ophthalmic vein and multiple signal voids within the left cavernous sinus, suggesting a direct CCF. Digital subtraction angiography confirmed the presence of a left post-traumatic CCF type A (Figure 3.1).
Figure 3.1. Digital subtraction angiography confirming the presence of a left post-traumatic CCF type A.
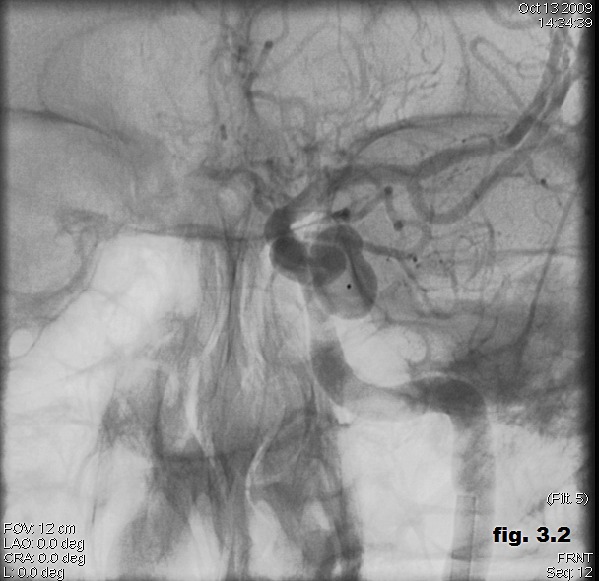
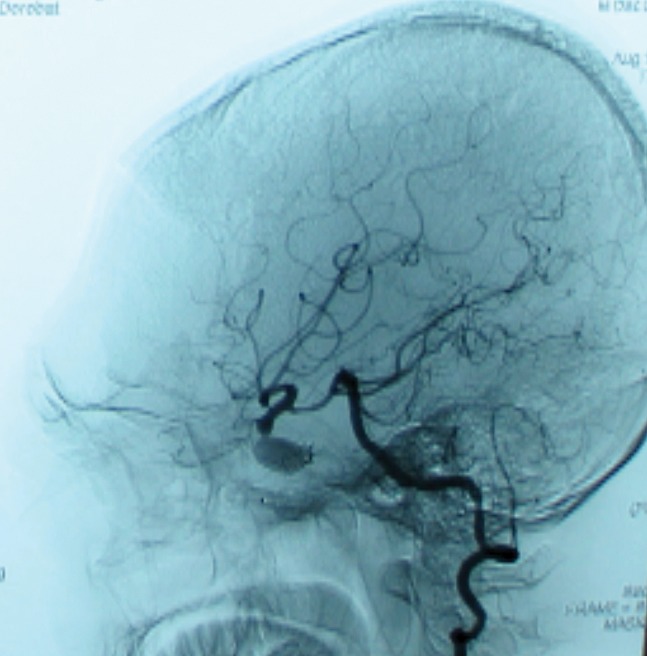
We performed transarterial embolization of the fistula on the left cavernous sinus by detachable balloon. It was introduced a guiding catheter into the left cervical ICA and a microcatheter with a tip mounted latex balloon selected the left cavernous sinus. The balloon was progressively inflated until the fistula was completely occluded (Figure 3.2).
Figure 3.2. Transarterial embolization of the fistula on the left cavernous sinus by detachable balloon.
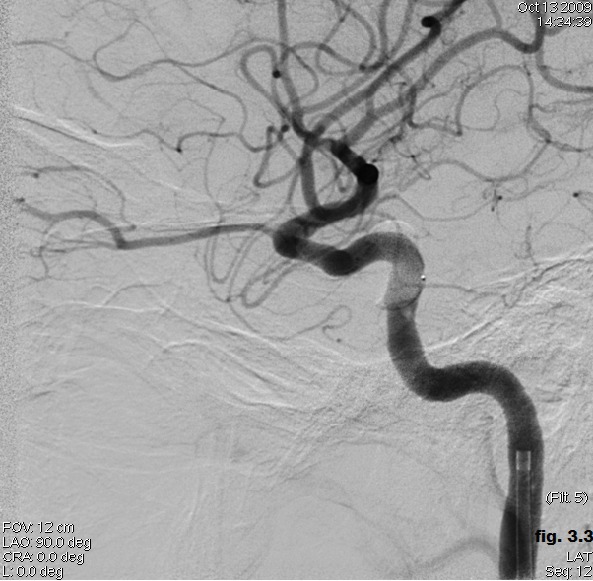
Postembolization angiography revealed that the fistula was successfully obliterated by detachable balloon (Figure 3.3).
Figure 3.3. Postembolization angiography (CCF obliterated by detachable balloon).
Case 4
We also present the case of 27 years, male who presented to our clinic 2 months after a boat accident with symptoms characterized by loss of visual sharpness, important chemosis, limitation of the ocular motility and ocular pain.
The imaging studies showed the presence of a right CCF fistula confirmed by the digital subtraction angiography as being a type A fistula.
With percutaneous access via the femoral artery there was implanted a detachable balloon by venous approach but the fistula was only partially occluded so we decided to use another detachable balloon guided into the right internal carotid artery and progressively inflated until complete occlusion was obtained (Figure 4).
Figure 4. Control angiogram - complete occlusion of the CCF using two detachable balloons.
Case 5
A 33 years old male who presented two months after a surgery for a left frontal traumatic injury with progressive bilateral exophthalmia (right eye more than left eye) and bilateral oculomotor nerve palsy. The neurologic exam showed that the patient was conscious, but somnolent, with right eye blindness, bilateral oculomotor nerve palsy and mild left hemiparesis. The cerebral CT scan showed post-surgery status without new tomodensitometric images.
The clinical picture raised the suspicion of a post-traumatic carotid-cavernous fistula. So, we performed a digital substraction angiography which showed the presence of the large size CCF with lack of intracerebral filling at the selective catheterization of the right ICA. Catheterization of the left ICA showed bilateral filling of the anterior and middle cerebral arteries and the right carotid compression leads to important, retrograde filling of the fistula through the anterior communicating artery. Catheterization of the left vertebral artery showed filling of the fistula through the right posterior communicating artery.
Using bilateral percutaneous access via both femoral arteries there were implanted a platinum coil (7x30 mm MATRIX) and a detachable balloon (9x17 mm BALT), the fistula being only partially occluded. One week later with a 6F guiding catheter positioned in the right ICA the complete occlusion of the fistula and right ICA was obtained using a another detachable balloon with filling of the right cerebral arteries through the communicating arteries (Figure 5). ❑
Figure 5. CCF occluded by coils and detachable balloons.
CONCLUSIONS
Endovascular treatment is the procedure of choice for the treatment of patients with traumatic CCF being less invasive than neurosurgical treatment. In these cases we presented, all of them having direct CCFs, which are high-flow fistulas, we had chosen as treatment option the occlusion of the fistulas mostly stent-grafts and detachable balloons due to the risks of migration of the embolic material in the distal circulation and serious secondary complications and secondary because coils are more expensive. As we described above direct CCF may be occluded using one or more detachable balloons, this being for the moment the method of choice for the treatment of CCF, with the goal of preserving the internal carotid artery (which is not always possible as we seen in the last cases) while the fistula is completely occluded. In the first two cases presented in this paper we choose the endovascular treatment by angioplasty with stent-graft, followed by repeated post-dilatations with a balloon with excellent results but there are several important issues concerning the placement of graft-stents in the cavernous part of internal carotid artery due to their stiffness. As we proceeded in the second case, if there is incomplete obliteration of the fistula it is safety to use a second stent-graft.
Our goal when using these techniques of endovascular treatment was the preservation of the carotid artery, which is not always possible, with obliteration of the fistula and clinical improvement.
References
- 1.Shownkeen H, Bova D, Origitano TC, et al. Carotid-Cavernous Fistulas: Pathogenesis and Routes of Approach to Endovascular Treatment. Skull Base. 2001;11:207–218. doi: 10.1055/s-2001-16609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chuman H, Trobe JD, Petty EM, et al. Spontaneous Direct Carotid-Caver¬nous Fistula in Ehlers-Danlos Syndrome Type IV: Two Case Reports and a Review of the Literature. J Neuro-Ophthalmol. 2002;22:75–81. doi: 10.1097/00041327-200206000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Barrow DL, Spector RH, Braun IF, et al. Classification and treatment of spontaneous carotid cavernous sinus fistulas. J Neurosurg. 1985;62:248–256. doi: 10.3171/jns.1985.62.2.0248. [DOI] [PubMed] [Google Scholar]
- 4.Keltner JL, Satterfield D, Dublin AB, et al. Diagnosis and Management of Carotid Cavernous Fistulas. Ophthalmology. 1987;94:1585–1600. doi: 10.1016/s0161-6420(87)33258-0. [DOI] [PubMed] [Google Scholar]
- 5.Ng PP, Higashida RT, Cullen S, et al. Endovascular strategies for carotid cavernous and intracerebral dural arteriovenous fistulas. Journal of Neurosurgery. 2003;15:1–6. doi: 10.3171/foc.2003.15.4.7. [DOI] [PubMed] [Google Scholar]
- 6.Lewis AI, Tomsick TA, Tew JM, et al. Long-term results in direct carotid cavernous fistula treatment with detachable balloon. J Neurosurg. 1996;84:400–404. doi: 10.3171/jns.1996.84.3.0400. [DOI] [PubMed] [Google Scholar]
- 7.Choi BJ, Hong Lee T, Won Kim C, et al. Endovascular Graft-Stent Placement for Treatment of Traumatic Carotid Cavernous Fistulas. J Korean Neurosurg Soc. 2009;46:572–576. doi: 10.3340/jkns.2009.46.6.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gomez F, Escobar W, Gomez AM, et al. Treatment of carotid cavernous fistulas using covered stents: midterm results in seven patients. AJNR Am J Neuroradiol. 2007;28:1762–1768. doi: 10.3174/ajnr.A0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klisch J, Huppertz HJ, Spetzger U, et al. Transvenous treatment of carotid cavernous and dural arteriovenous fistulae: results for 31 patients and review of the literature. Neurosurgery. 2003;53:836–856. doi: 10.1227/01.neu.0000083551.26295.ab. [DOI] [PubMed] [Google Scholar]
- 10.Luo CB, Teng MM, Yen DH, et al. Endovascular embolization of recurrent traumatic carotid-cavernous fistulas managed previously with detachable balloons. J Trauma. 2004;56:1214–1220. doi: 10.1097/01.ta.0000131213.93205.57. [DOI] [PubMed] [Google Scholar]
- 11.Remonda L, Frigerio SB, Buhler R, et al. Transvenous coil treatment of a type A carotid cavernous fistula in association with transarterial trispan coil protection. AJNR Am J Neuroradiol. 2004;25:611–613. [PMC free article] [PubMed] [Google Scholar]
- 12.Halbach VV, Higashida RT, Hieshima GB, et al. Transvenous embolization of direct carotid cavernous fistulas. AJNR. 1988;9:741–747. [PMC free article] [PubMed] [Google Scholar]
- 13.Ogilvy CS, Stieg PE, Awad I, et al. Recommendations for the Management of Intracranial Arteriovenous Malformations. Stroke. 2001;32:1458–1458. doi: 10.1161/01.str.32.6.1458. [DOI] [PubMed] [Google Scholar]
- 14.Troffkin NA, Given CA. Combined transarterial N-butyl cyanoacrylate and coil embolization of direct carotid–cavernous fistulas. JNSPG. 2007:106,no5–106,no5. doi: 10.3171/jns.2007.106.5.903. [DOI] [PubMed] [Google Scholar]
- 15.Saatci I, Cekirge HS, Ozturk MH, et al. Treatment of internal carotid artery aneurysms with a covered stent: experience in 24 patients with mid-term follow-up results. AJNR Am J Neuroradiol. 2004;25:1742–1749. [PMC free article] [PubMed] [Google Scholar]
- 16.Maras D, Lioupis C, Magoufis G, et al. Covered stent-graft treatment of traumatic internal carotid artery pseudoaneurysms : a review. Cardiovasc Intervent Radiol. 2006;29:958–968. doi: 10.1007/s00270-005-0367-7. [DOI] [PubMed] [Google Scholar]
- 17.Archondakis E, Pero G, Valvassori L, et al. Angiographic follow-up of traumatic carotid cavernous fistulas treated with endovascular stent graft placement. AJNR Am J Neuroradiol. 2007;28:342–347. [PMC free article] [PubMed] [Google Scholar]
- 18.Felber S, Henkes H, Weber W, et al. Treatment of extracranial and intracranial aneurysms and arteriovenous fistulae using stent grafts. Neurosurgery. 2004;55:631–638. doi: 10.1227/01.neu.0000134455.02947.1f. [DOI] [PubMed] [Google Scholar]