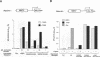Abstract
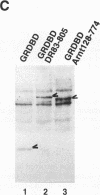
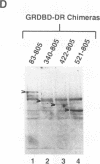
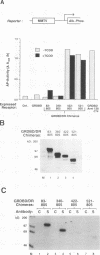
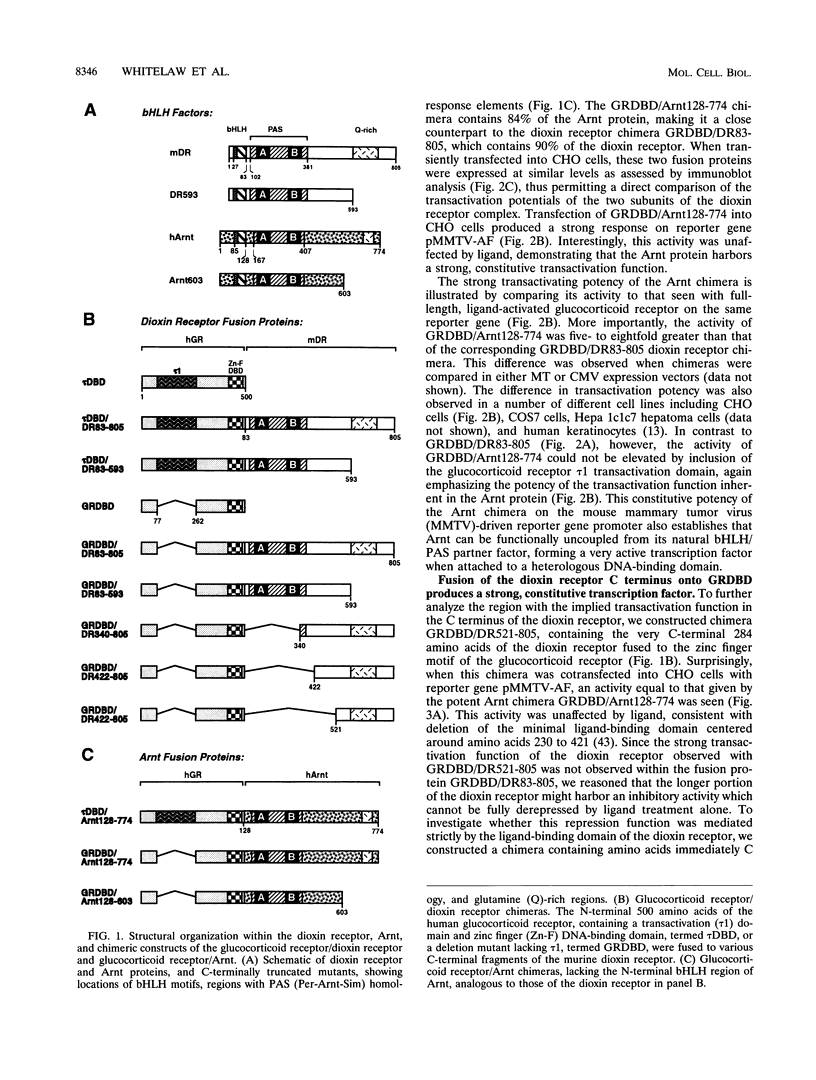
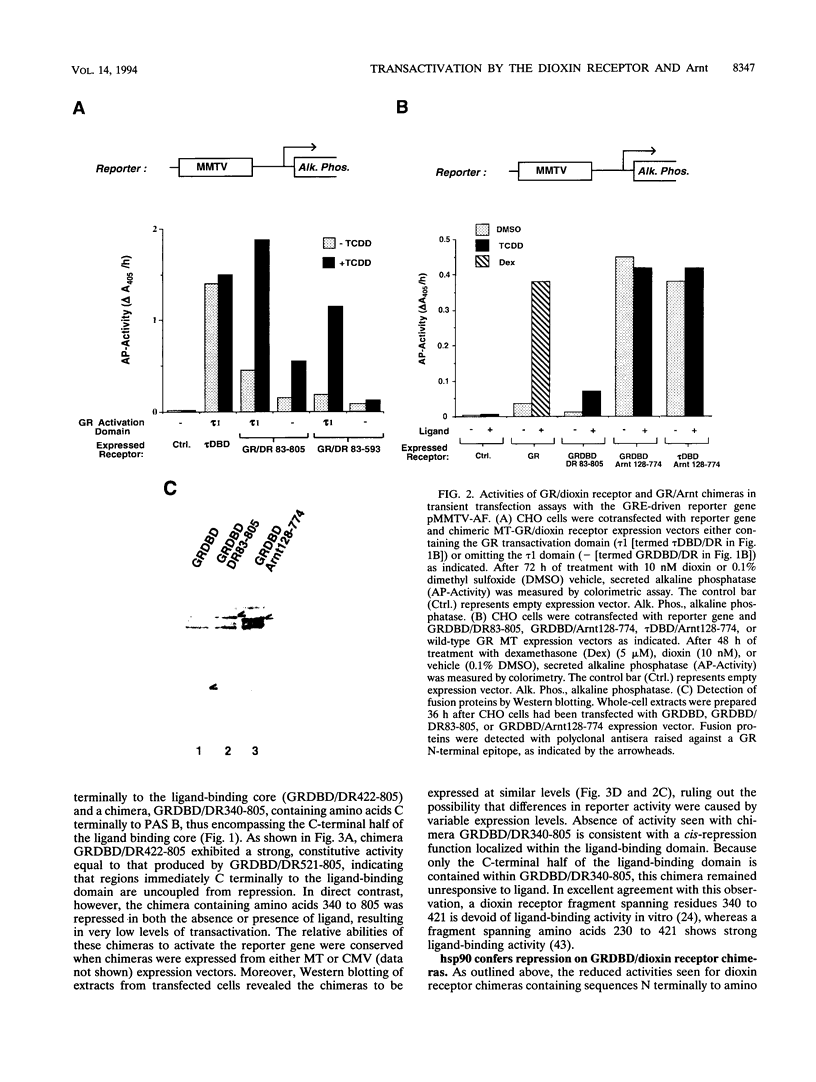
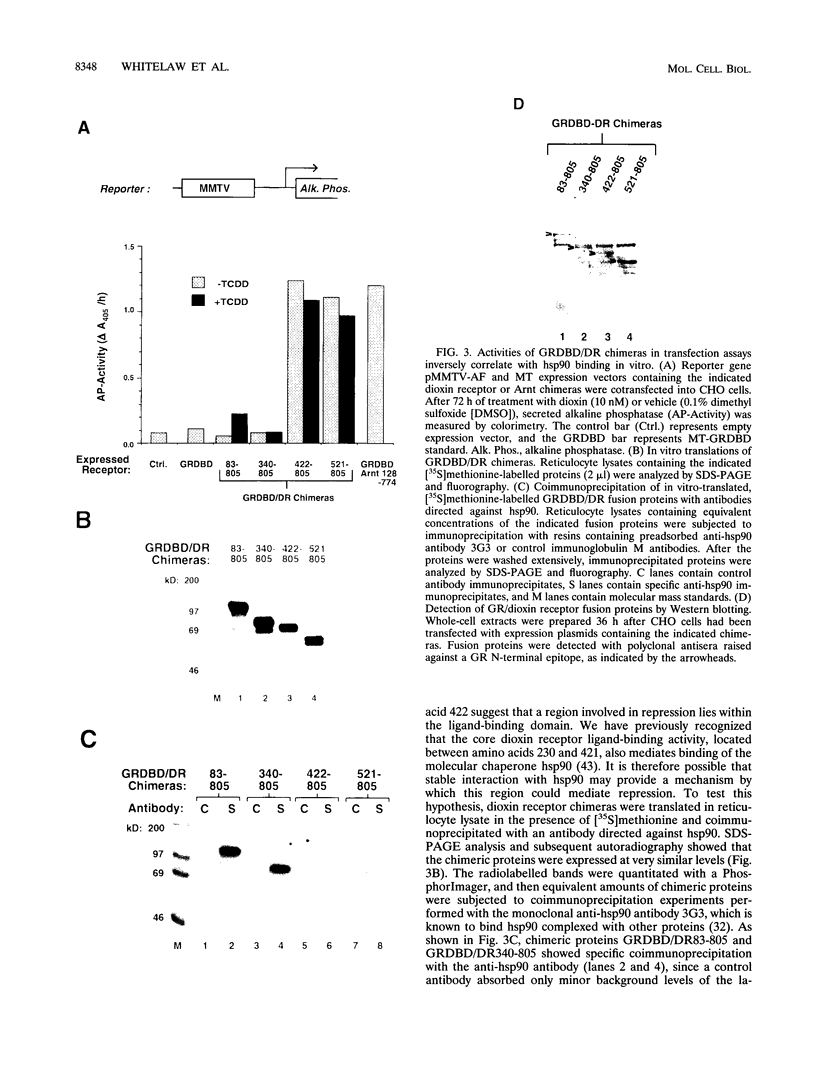
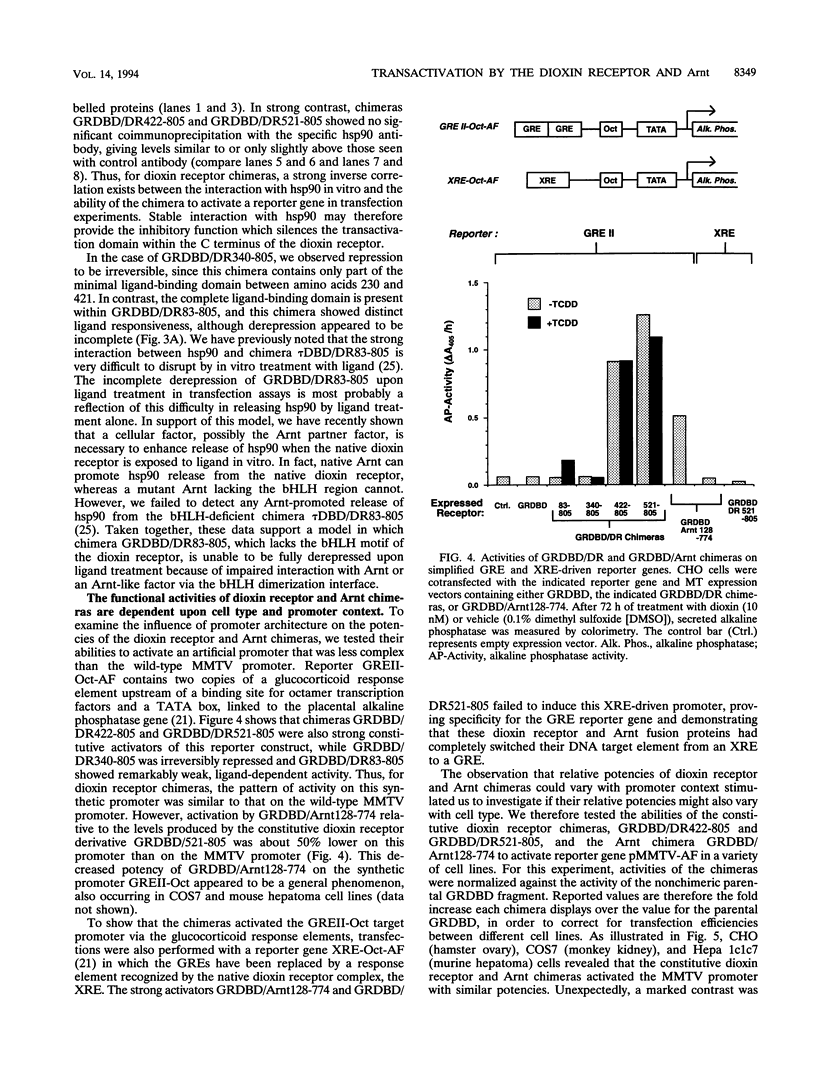
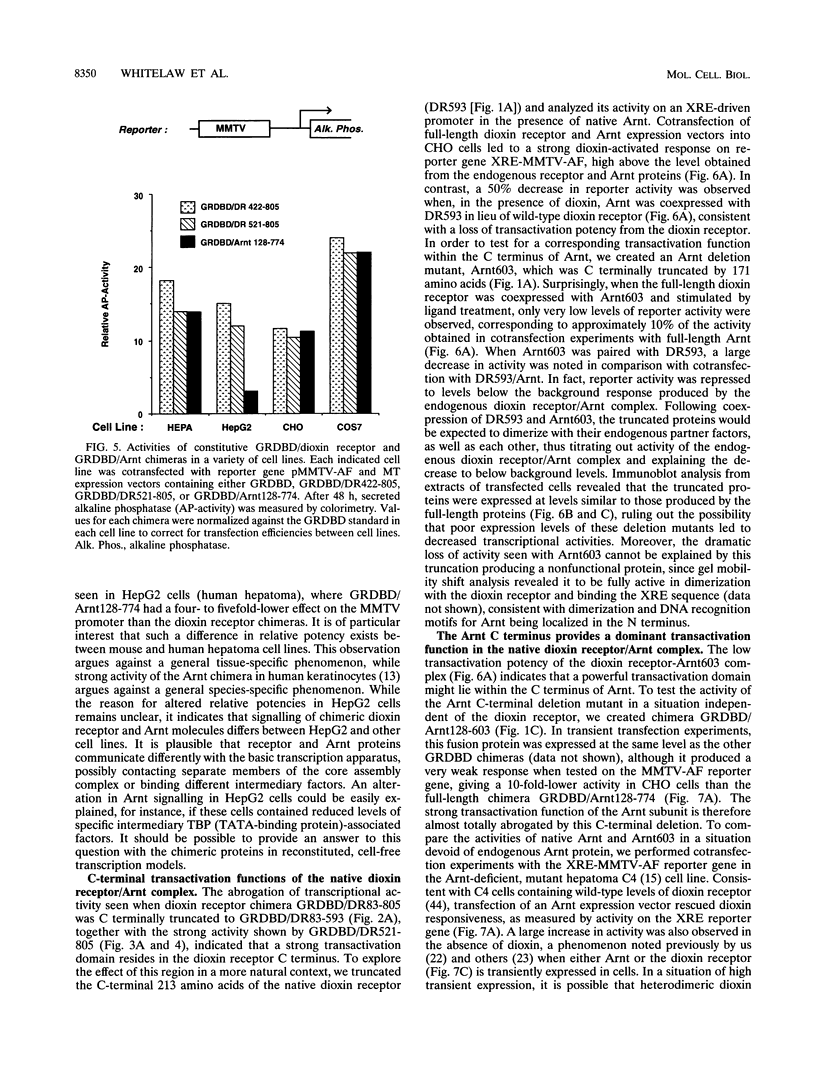
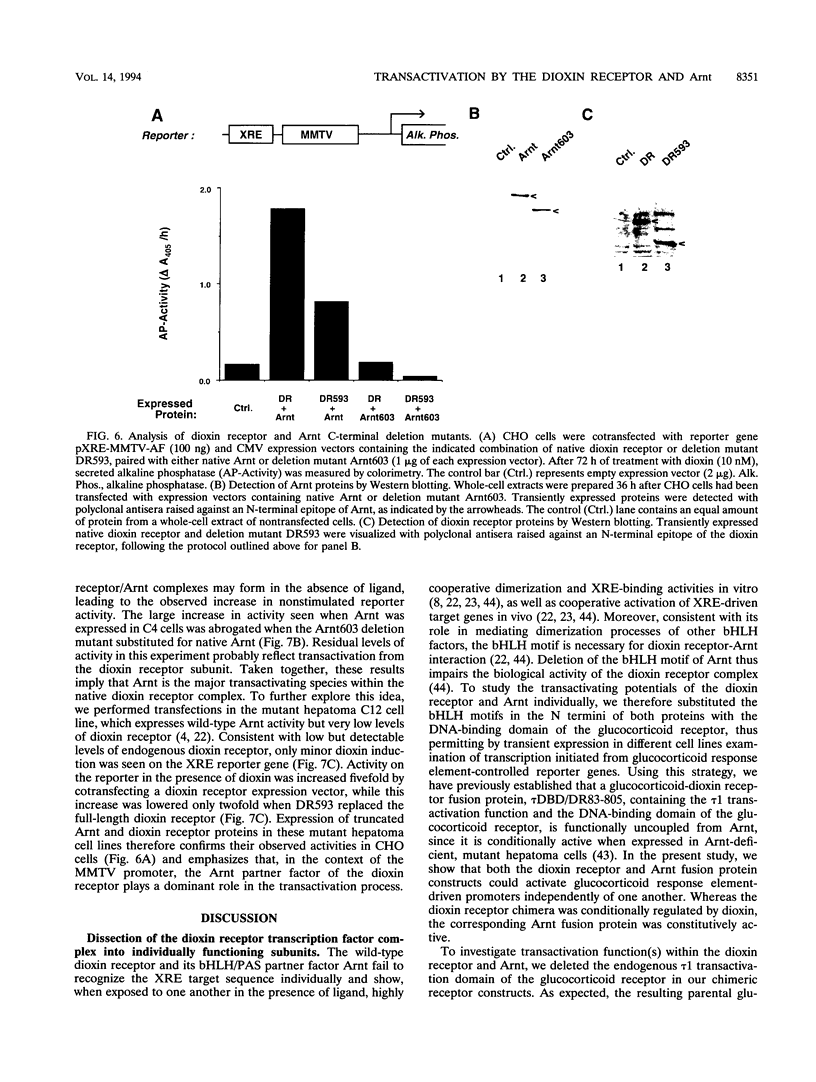
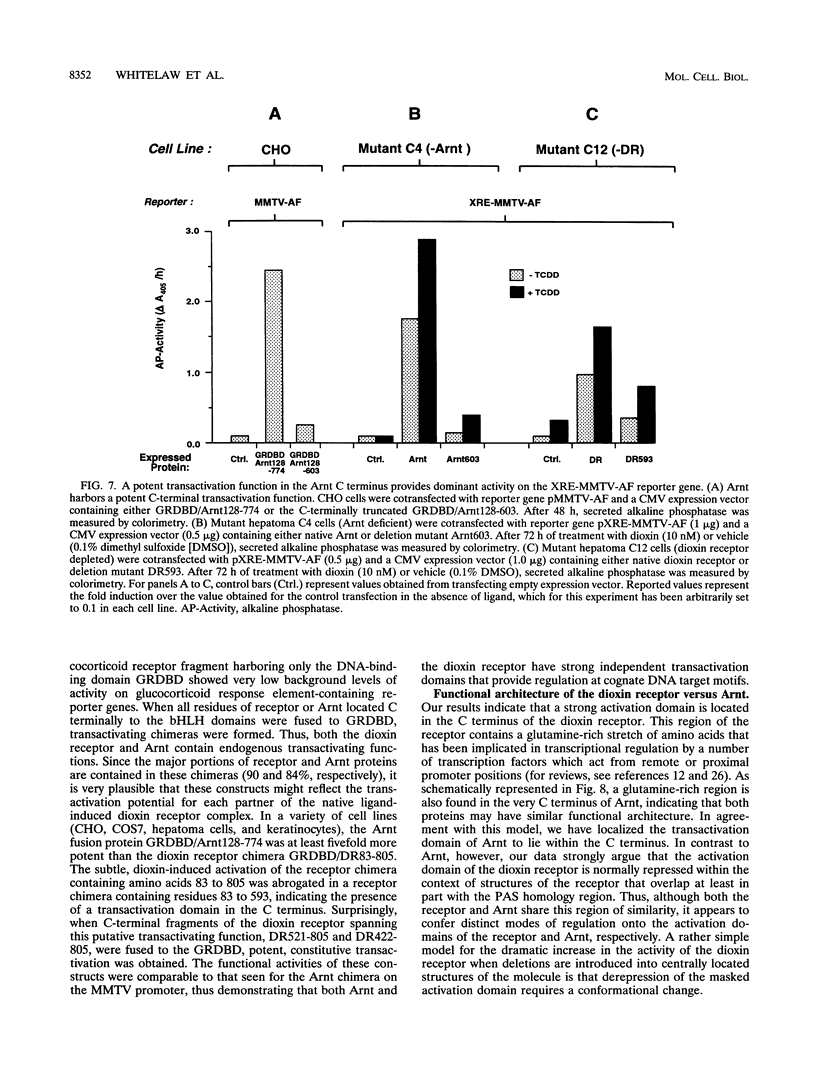
Gene regulation by dioxins is mediated via the dioxin receptor, a ligand-dependent basic helix-loop-helix (bHLH)/PAS transcription factor. The latent dioxin receptor responds to dioxin signalling by forming an activated heterodimeric complex with a specific bHLH partner, Arnt, an essential process for target DNA recognition. We have analyzed the transactivating potential within this heterodimeric complex by dissecting it into individual subunits, replacing the dimerization and DNA-binding bHLH motifs with heterologous zinc finger DNA-binding domains. The uncoupled Arnt chimera, maintaining 84% of Arnt residues, forms a potent and constitutive transcription factor. Chimeric proteins show that the dioxin receptor also harbors a strong transactivation domain in the C terminus, although this activity was silenced by inclusion of 82 amino acids from the central ligand-binding portion of the dioxin receptor. This central repression region conferred binding of the molecular chaperone hsp90 upon otherwise constitutive chimeras in vitro, indicating that hsp90 has the ability to mediate a cis-repressive function on distant transactivation domains. Importantly, when the ligand-binding domain of the dioxin receptor remained intact, the ability of this hsp90-binding activity to confer repression became conditional rather than irreversible. Our data are consistent with a model in which crucial activities of the dioxin receptor, such as dimerization with Arnt and transactivation, are conditionally repressed by the central ligand- and-hsp90-binding region of the receptor. In contrast, the Arnt protein appears to be free from any repressive activity. Moreover, within the context of the dioxin response element (xenobiotic response element), the C terminus of Arnt conferred a potent, dominating transactivation function onto the native bHLH heterodimeric complex. Finally, the relative transactivation potencies of the individual dioxin receptor and Arnt chimeras varied with cell type and promoter architecture, indicating that the mechanisms for transcriptional activation may differ between these two subunits and that in the native complex the transactivation pathway may be dependent upon cell-specific and promoter contexts.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berghard A., Gradin K., Pongratz I., Whitelaw M., Poellinger L. Cross-coupling of signal transduction pathways: the dioxin receptor mediates induction of cytochrome P-450IA1 expression via a protein kinase C-dependent mechanism. Mol Cell Biol. 1993 Jan;13(1):677–689. doi: 10.1128/mcb.13.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohen S. P., Yamamoto K. R. Isolation of Hsp90 mutants by screening for decreased steroid receptor function. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):11424–11428. doi: 10.1073/pnas.90.23.11424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresnick E. H., Dalman F. C., Sanchez E. R., Pratt W. B. Evidence that the 90-kDa heat shock protein is necessary for the steroid binding conformation of the L cell glucocorticoid receptor. J Biol Chem. 1989 Mar 25;264(9):4992–4997. [PubMed] [Google Scholar]
- Burbach K. M., Poland A., Bradfield C. A. Cloning of the Ah-receptor cDNA reveals a distinctive ligand-activated transcription factor. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):8185–8189. doi: 10.1073/pnas.89.17.8185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrier F., Owens R. A., Nebert D. W., Puga A. Dioxin-dependent activation of murine Cyp1a-1 gene transcription requires protein kinase C-dependent phosphorylation. Mol Cell Biol. 1992 Apr;12(4):1856–1863. doi: 10.1128/mcb.12.4.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielian P. S., White R., Lees J. A., Parker M. G. Identification of a conserved region required for hormone dependent transcriptional activation by steroid hormone receptors. EMBO J. 1992 Mar;11(3):1025–1033. doi: 10.1002/j.1460-2075.1992.tb05141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis M., Cuthill S., Wikström A. C., Poellinger L., Gustafsson J. A. Association of the dioxin receptor with the Mr 90,000 heat shock protein: a structural kinship with the glucocorticoid receptor. Biochem Biophys Res Commun. 1988 Sep 15;155(2):801–807. doi: 10.1016/s0006-291x(88)80566-7. [DOI] [PubMed] [Google Scholar]
- Dolwick K. M., Swanson H. I., Bradfield C. A. In vitro analysis of Ah receptor domains involved in ligand-activated DNA recognition. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8566–8570. doi: 10.1073/pnas.90.18.8566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ema M., Sogawa K., Watanabe N., Chujoh Y., Matsushita N., Gotoh O., Funae Y., Fujii-Kuriyama Y. cDNA cloning and structure of mouse putative Ah receptor. Biochem Biophys Res Commun. 1992 Apr 15;184(1):246–253. doi: 10.1016/0006-291x(92)91185-s. [DOI] [PubMed] [Google Scholar]
- Fujii-Kuriyama Y., Imataka H., Sogawa K., Yasumoto K., Kikuchi Y. Regulation of CYP1A1 expression. FASEB J. 1992 Jan 6;6(2):706–710. doi: 10.1096/fasebj.6.2.1537460. [DOI] [PubMed] [Google Scholar]
- Fujisawa-Sehara A., Sogawa K., Yamane M., Fujii-Kuriyama Y. Characterization of xenobiotic responsive elements upstream from the drug-metabolizing cytochrome P-450c gene: a similarity to glucocorticoid regulatory elements. Nucleic Acids Res. 1987 May 26;15(10):4179–4191. doi: 10.1093/nar/15.10.4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber H. P., Seipel K., Georgiev O., Höfferer M., Hug M., Rusconi S., Schaffner W. Transcriptional activation modulated by homopolymeric glutamine and proline stretches. Science. 1994 Feb 11;263(5148):808–811. doi: 10.1126/science.8303297. [DOI] [PubMed] [Google Scholar]
- Gradin K., Whitelaw M. L., Toftgård R., Poellinger L., Berghard A. A tyrosine kinase-dependent pathway regulates ligand-dependent activation of the dioxin receptor in human keratinocytes. J Biol Chem. 1994 Sep 23;269(38):23800–23807. [PubMed] [Google Scholar]
- Gronemeyer H. Transcription activation by estrogen and progesterone receptors. Annu Rev Genet. 1991;25:89–123. doi: 10.1146/annurev.ge.25.120191.000513. [DOI] [PubMed] [Google Scholar]
- Hoffman E. C., Reyes H., Chu F. F., Sander F., Conley L. H., Brooks B. A., Hankinson O. Cloning of a factor required for activity of the Ah (dioxin) receptor. Science. 1991 May 17;252(5008):954–958. doi: 10.1126/science.1852076. [DOI] [PubMed] [Google Scholar]
- Huang Z. J., Edery I., Rosbash M. PAS is a dimerization domain common to Drosophila period and several transcription factors. Nature. 1993 Jul 15;364(6434):259–262. doi: 10.1038/364259a0. [DOI] [PubMed] [Google Scholar]
- Jakob U., Buchner J. Assisting spontaneity: the role of Hsp90 and small Hsps as molecular chaperones. Trends Biochem Sci. 1994 May;19(5):205–211. doi: 10.1016/0968-0004(94)90023-x. [DOI] [PubMed] [Google Scholar]
- Jan Y. N., Jan L. Y. HLH proteins, fly neurogenesis, and vertebrate myogenesis. Cell. 1993 Dec 3;75(5):827–830. doi: 10.1016/0092-8674(93)90525-u. [DOI] [PubMed] [Google Scholar]
- Kadesch T. Consequences of heteromeric interactions among helix-loop-helix proteins. Cell Growth Differ. 1993 Jan;4(1):49–55. [PubMed] [Google Scholar]
- Kang K. I., Devin J., Cadepond F., Jibard N., Guiochon-Mantel A., Baulieu E. E., Catelli M. G. In vivo functional protein-protein interaction: nuclear targeted hsp90 shifts cytoplasmic steroid receptor mutants into the nucleus. Proc Natl Acad Sci U S A. 1994 Jan 4;91(1):340–344. doi: 10.1073/pnas.91.1.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason G. G., Witte A. M., Whitelaw M. L., Antonsson C., McGuire J., Wilhelmsson A., Poellinger L., Gustafsson J. A. Purification of the DNA binding form of dioxin receptor. Role of the Arnt cofactor in regulation of dioxin receptor function. J Biol Chem. 1994 Feb 11;269(6):4438–4449. [PubMed] [Google Scholar]
- Matsushita N., Sogawa K., Ema M., Yoshida A., Fujii-Kuriyama Y. A factor binding to the xenobiotic responsive element (XRE) of P-4501A1 gene consists of at least two helix-loop-helix proteins, Ah receptor and Arnt. J Biol Chem. 1993 Oct 5;268(28):21002–21006. [PubMed] [Google Scholar]
- McGuire J., Whitelaw M. L., Pongratz I., Gustafsson J. A., Poellinger L. A cellular factor stimulates ligand-dependent release of hsp90 from the basic helix-loop-helix dioxin receptor. Mol Cell Biol. 1994 Apr;14(4):2438–2446. doi: 10.1128/mcb.14.4.2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P. J., Tjian R. Transcriptional regulation in mammalian cells by sequence-specific DNA binding proteins. Science. 1989 Jul 28;245(4916):371–378. doi: 10.1126/science.2667136. [DOI] [PubMed] [Google Scholar]
- Miyata Y., Yahara I. The 90-kDa heat shock protein, HSP90, binds and protects casein kinase II from self-aggregation and enhances its kinase activity. J Biol Chem. 1992 Apr 5;267(10):7042–7047. [PubMed] [Google Scholar]
- Nambu J. R., Lewis J. O., Wharton K. A., Jr, Crews S. T. The Drosophila single-minded gene encodes a helix-loop-helix protein that acts as a master regulator of CNS midline development. Cell. 1991 Dec 20;67(6):1157–1167. doi: 10.1016/0092-8674(91)90292-7. [DOI] [PubMed] [Google Scholar]
- Nemoto T., Ohara-Nemoto Y., Denis M., Gustafsson J. A. The transformed glucocorticoid receptor has a lower steroid-binding affinity than the nontransformed receptor. Biochemistry. 1990 Feb 20;29(7):1880–1886. doi: 10.1021/bi00459a031. [DOI] [PubMed] [Google Scholar]
- Okino S. T., Pendurthi U. R., Tukey R. H. Phorbol esters inhibit the dioxin receptor-mediated transcriptional activation of the mouse Cyp1a-1 and Cyp1a-2 genes by 2,3,7,8-tetrachlorodibenzo-p-dioxin. J Biol Chem. 1992 Apr 5;267(10):6991–6998. [PubMed] [Google Scholar]
- Perdew G. H. Association of the Ah receptor with the 90-kDa heat shock protein. J Biol Chem. 1988 Sep 25;263(27):13802–13805. [PubMed] [Google Scholar]
- Perdew G. H., Whitelaw M. L. Evidence that the 90-kDa heat shock protein (HSP90) exists in cytosol in heteromeric complexes containing HSP70 and three other proteins with Mr of 63,000, 56,000, and 50,000. J Biol Chem. 1991 Apr 15;266(11):6708–6713. [PubMed] [Google Scholar]
- Picard D., Khursheed B., Garabedian M. J., Fortin M. G., Lindquist S., Yamamoto K. R. Reduced levels of hsp90 compromise steroid receptor action in vivo. Nature. 1990 Nov 8;348(6297):166–168. doi: 10.1038/348166a0. [DOI] [PubMed] [Google Scholar]
- Picard D., Yamamoto K. R. Two signals mediate hormone-dependent nuclear localization of the glucocorticoid receptor. EMBO J. 1987 Nov;6(11):3333–3340. doi: 10.1002/j.1460-2075.1987.tb02654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollenz R. S., Sattler C. A., Poland A. The aryl hydrocarbon receptor and aryl hydrocarbon receptor nuclear translocator protein show distinct subcellular localizations in Hepa 1c1c7 cells by immunofluorescence microscopy. Mol Pharmacol. 1994 Mar;45(3):428–438. [PubMed] [Google Scholar]
- Pongratz I., Mason G. G., Poellinger L. Dual roles of the 90-kDa heat shock protein hsp90 in modulating functional activities of the dioxin receptor. Evidence that the dioxin receptor functionally belongs to a subclass of nuclear receptors which require hsp90 both for ligand binding activity and repression of intrinsic DNA binding activity. J Biol Chem. 1992 Jul 5;267(19):13728–13734. [PubMed] [Google Scholar]
- Pratt W. B. The role of heat shock proteins in regulating the function, folding, and trafficking of the glucocorticoid receptor. J Biol Chem. 1993 Oct 15;268(29):21455–21458. [PubMed] [Google Scholar]
- Probst M. R., Reisz-Porszasz S., Agbunag R. V., Ong M. S., Hankinson O. Role of the aryl hydrocarbon receptor nuclear translocator protein in aryl hydrocarbon (dioxin) receptor action. Mol Pharmacol. 1993 Sep;44(3):511–518. [PubMed] [Google Scholar]
- Reyes H., Reisz-Porszasz S., Hankinson O. Identification of the Ah receptor nuclear translocator protein (Arnt) as a component of the DNA binding form of the Ah receptor. Science. 1992 May 22;256(5060):1193–1195. doi: 10.1126/science.256.5060.1193. [DOI] [PubMed] [Google Scholar]
- Smith D. F., Toft D. O. Steroid receptors and their associated proteins. Mol Endocrinol. 1993 Jan;7(1):4–11. doi: 10.1210/mend.7.1.8446107. [DOI] [PubMed] [Google Scholar]
- Takahashi J. S. Circadian clock genes are ticking. Science. 1992 Oct 9;258(5080):238–240. doi: 10.1126/science.1384127. [DOI] [PubMed] [Google Scholar]
- Tate B. F., Allenby G., Janocha R., Kazmer S., Speck J., Sturzenbecker L. J., Abarzúa P., Levin A. A., Grippo J. F. Distinct binding determinants for 9-cis retinoic acid are located within AF-2 of retinoic acid receptor alpha. Mol Cell Biol. 1994 Apr;14(4):2323–2330. doi: 10.1128/mcb.14.4.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitelaw M. L., Göttlicher M., Gustafsson J. A., Poellinger L. Definition of a novel ligand binding domain of a nuclear bHLH receptor: co-localization of ligand and hsp90 binding activities within the regulable inactivation domain of the dioxin receptor. EMBO J. 1993 Nov;12(11):4169–4179. doi: 10.1002/j.1460-2075.1993.tb06101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitelaw M., Pongratz I., Wilhelmsson A., Gustafsson J. A., Poellinger L. Ligand-dependent recruitment of the Arnt coregulator determines DNA recognition by the dioxin receptor. Mol Cell Biol. 1993 Apr;13(4):2504–2514. doi: 10.1128/mcb.13.4.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiech H., Buchner J., Zimmermann R., Jakob U. Hsp90 chaperones protein folding in vitro. Nature. 1992 Jul 9;358(6382):169–170. doi: 10.1038/358169a0. [DOI] [PubMed] [Google Scholar]
- Wilhelmsson A., Cuthill S., Denis M., Wikström A. C., Gustafsson J. A., Poellinger L. The specific DNA binding activity of the dioxin receptor is modulated by the 90 kd heat shock protein. EMBO J. 1990 Jan;9(1):69–76. doi: 10.1002/j.1460-2075.1990.tb08081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Lindquist S. Heat-shock protein hsp90 governs the activity of pp60v-src kinase. Proc Natl Acad Sci U S A. 1993 Aug 1;90(15):7074–7078. doi: 10.1073/pnas.90.15.7074. [DOI] [PMC free article] [PubMed] [Google Scholar]