Abstract
Introduction
Low-energy shockwave therapy (LESWT) has been shown to improve erectile function in patients suffering from diabetes mellitus (DM)-associated erectile dysfunction (ED). However, the underlying mechanism remains unknown.
Aim
To investigate whether LESWT can ameliorate DM-associated ED in a rat model, and examine the associated changes in the erectile tissues.
Methods
Newborn male rats were intraperitoneally injected with 5-ethynyl-2-deoxyuridine (EdU, 50mg/kg) for the purpose of tracking endogenous mesenchymal stem cells (MSCs). Eight weeks later, 8 of these rats were randomly chosen to serve as normal control (N group). The remaining rats were injected intraperitoneally with 60 mg/kg of streptozotocin (STZ) to induce DM. Eight of these rats were randomly chosen to serve as DM control (DM group) while another 8 rats were subject to shockwave treatment (DM+SW group). Each rat in the DM+SE group received 300 shocks at energy level of 0.1mJ/mm2 and frequency of 120/min. This procedure was repeated three times a week for two weeks. Another two weeks later, all 24 rats were evaluated for erectile function by intracavernous pressure (ICP) measurement. Afterward, their penile tissues were examined by histology.
Main Outcome Measures
Erectile function was measurement by ICP. Neuronal nitric oxide synthase (nNOS)-positive nerves and the endothelium were examined by immunofluorescence (IF) staining. Smooth muscle and MSCs were examined by phalloidin and EdU staining, respectively.
Results
STZ treatment caused a significant decrease in erectile function and in the number of nNOS-positive nerves and in endothelial and smooth muscle contents. These DM-associated deficits were all partially but significantly reversed by LESWT. MSCs (EdU+ cells) were significantly more numerous in DM+SW than in DM rats.
Conclusions
LESWT can partially ameliorate DM-associated ED by promoting regeneration of nNOS-positive nerves, endothelium, and smooth muscle in the penis. These beneficial effects appear to be mediated by recruitment of endogenous MSCs.
Keywords: Low-energy shockwave, Diabetes mellitus, Erectile dysfunction
Introduction
Erectile dysfunction (ED) is a prevailing health problem that seriously impacts the quality of life of men and their spouses or partners [1]. While the majority of ED patients can be satisfactorily treated with PDE5 inhibitors, a substantial population (30-40%) cannot [2]. This includes patients who are intolerant to PDE5 inhibitors’ side effects, taking nitrate medication for angina, or having certain types of ED refractory to PDE5 inhibitors. In particular, diabetes mellitus (DM) and surgery-induced cavernous nerve injuries (mainly due to radical prostatectomy) are currently the most common causes of refractory ED [2]. To treat these types of ED, one of the proposed strategies is low-energy shockwave therapy (LESWT), as seen in recently published studies [3-5] and ongoing clinical trials (NCT01317693, NCT01274923, NCT01442077, and NCT01317680 at http://clinicaltrials.gov). In one study involving 29 severe ED patients, LESWT was found to substantially increase erectile function scores with concomitant improvement of penile hemodynamics [3]. Noteworthy is that the majority of these patients (21 out of 29) were diabetic, and thus their positive response to LESWT raises the question whether LESWT is specifically effective for treating DM-associated ED. While the answer awaits further clinical studies, it should be pointed out that, despite successful demonstration in clinical trials, LESWT as an ED treatment modality has not been investigated at the basic science level and has no known mechanistic basis.
LESWT has been investigated in animal models of heart failure [6], coronary arterial disease [7], ischemic myocardial dysfunction [8], ischemic tissue necrosis [9], chronic hind limb ischemia [10], and bone defects [11]. The outcomes invariably point to induction of angiogenesis as one of the underlying mechanisms for LESWT’s therapeutic effects. In addition, one of these studies also identified recruitment of mesenchymal stem cells (MSCs) as a possible mechanism [11]. In ED field, angiogenesis is known to play important roles for the therapeutic effects of growth factors and gene therapies [12-14]. And both exogenously applied and endogenously recruited MSCs have been shown to enhance recovery of erectile function in ED animal models [15, 16]. Thus, the observed therapeutic effects of LESWT in ED patients are likely mediated by angiogenesis and MSC recruitment. In the present study we tested this hypothesis; specifically, we investigated the effects of LESWT on erectile function and related tissue structures in a streptozotocin (STZ)-induced DM rat model. We also examined whether LESWT enhanced MSC recruitment by using the label-retaining cell (LRC) strategy [17] with EdU being the thymidine analog for such labeling [18].
Materials and methods
Animals
All animal experiments in this study were approved by the Institutional Animal Care and Use Committee at our institution. Pregnant Sprague-Dawley rats were purchased from Charles River Laboratories (Wilmington, MA) for the investigation of childbirth-related urinary incontinence in separate projects. Their newborn male rats were used for this study. For the purpose of tracking endogenous MSCs, each pup received intraperitoneal injection of EdU (50 mg/kg, Invitrogen, Carlsbad, CA) immediately after birth, as described previously [18-20]. At 8 weeks of age, 8 of these rats were randomly selected to serve as normal control (N). The remaining rats were each injected intraperitoneally with 60 mg/kg of STZ (Sigma-Aldrich, St. Louis, MO), and their blood glucose level was monitored weekly by checking tail vein blood with Accutrend strip (Roche Diagnostics, Indianapolis, IN). Rats with fasting blood glucose of ≥200 mg/dl were designated as diabetic and selected for further tests. A total of 16 such rats were equally randomized into a diabetic group (DM) and a diabetic plus LESWT group (DM+SW).
Shockwave treatment
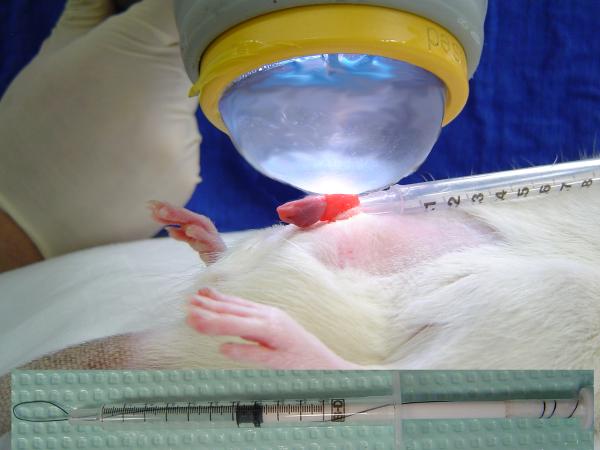
Four weeks post-STZ injection, rats in the DM+SW group were treated with shockwaves as depicted in Fig. 1 and explained in the following. Under anesthesia, each rat was placed in a supine position, its lower abdomen shaved, and its penis drawn out of the prepuce and held in place with a loop made of suture line and syringe. After application of ultrasound gel (Aquasonic; Parker Laboratories, Inc, Fairfield, NJ) on the penis, a shockwave applicator (DermaGold, MTS Europe GmbH, Konstanz, Germany) was placed in contact with the penis, and a total of 300 shocks were delivered at energy level of 0.1mJ/mm2 and frequency of 120/min. This procedure was repeated three times a week for two weeks, and the entire treatment course is comparable to clinical shockwave treatment for ED patients [3-5]. Due to the fact that DermaGold is clinically approved to treat superficial wounds, its delivered shockwave is expected to penetrate a few centimeters (probably the thickness of a rat penis) in the contacted area.
Figure 1. Shockwave application to the rat penis.
Under anesthesia, the penis was drawn out of the prepuce, held in place with a loop made of suture line and syringe (shown in inset), applied with ultrasound gel, and shockwave-treated.
Erectile function evaluation
Two weeks after the final shockwave treatment for rats in the DM+SW group, all 24 rats (in N, DM, and DM+SW groups) were tested for erectile function by measuring intracavernous pressure (ICP) in response to electrostimulation of cavernous nerves. Briefly, under ketamine (100mg/kg) and midazolam (5mg/kg) anesthesia, the cavernous nerves were exposed via midline laparotomy. The corpus cavernosum was cannulated with a heparinized (200U/ml) 25G needle connected to a pressure transducer (Utah Medical Products, Midvale, UT). The stimulus parameters were 20Hz, pulse width of 0.2 ms, and duration of 50s with three different current settings: 0.5 mA, 1.0 mA and 1.5 mA. The maximum increase of ICP of three stimuli per side was selected for statistical analysis in each animal. ICP was normalized to mean arterial pressure (MAP), which was recorded using a 25G needle inserted into the aortic bifurcation.
Histology
At the conclusion of erectile function evaluation, the rats were sacrificed and their penis harvested for histology. The penis (mid-shaft portion) was fixed with 2% formaldehyde and 0.002% picric acid in 0.1M PBS for 4 hours, followed by immersion in 30% sucrose in PBS overnight at 4°C. The fixed tissue was then embedded in optimal cutting temperature compound (Sakura Finetek, Torrance, CA), cut into 5 μm-thick sections, mounted on glass slides (3 sections per slide), and subjected to immunofluorescent (IF) and EdU staining. For IF staining, the slides were placed in 0.3% H2O2/methanol for 10 min, washed twice in PBS for 5 min and incubated with 3% horse serum in PBS/0.3% Triton X-100 for 30 min at room temperature. After draining this solution from the tissue section, the slides were incubated at room temperature with rabbit anti-nNOS (1:100, SC-648, Santa Cruz Biotechnology, Santa Cruz, CA) or mouse anti-rat endothelial cell antigen (RECA; 1:500; MCA-970R, AbD Serotec, Raleigh, NC) antibody overnight. Control tissue sections were similarly prepared except no primary antibody was added. After rinses with PBS, the sections were incubated with Alexa-488- or Alexa-594-conjugated secondary antibody (Invitrogen). Smooth muscle was stained by incubation with Alexa-488-conjugated phalloidin (Invitrogen) for 20 min at room temperature. Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI; Invitrogen). For tracking EdU-positive cells, tissue sections were incubated with Click-IT reaction cocktail (Invitrogen) for 30 min at room temperature.
Image analysis and quantification
The stained tissues were examined with Nikon Eclipse E600 fluorescence microscope and photographed with Retiga 1300 Q-imaging camera using the ACT-1 software (Nikon Instruments, Melville, NY). For evaluation of cavernous smooth muscle and endothelial contents, two fields (both sides of the corpus cavernosum at 200× magnification) on each tissue section were photographed. For evaluation of arterial endothelial content, two arteries within the corpus cavernosum at 1000× magnification on each tissue section were photographed. For evaluation of dorsal nerve nNOS expression, two fields (the two largest dorsal nerve branches at 400× magnification) on each tissue section were photographed. For evaluation of nNOS expression around dorsal arteries, the two dorsal arteries on each tissue section at 400× were photographed. For evaluation of nNOS expression in the corpus cavernosum, two fields (both sides of the corpus cavernosum at 400× magnification) on each tissue section were photographed. In each of these photographic recordings the images were generated in green, red, and blue channels, and these single-color images were then superimposed to generate the multi-color figures. For quantification, the single-color images were analyzed with Image-Plus 5.1 software (Media Cybernetics, Bethesda, MD). To quantify cavernous endothelium, RECA-stained area (in red) was measured and expressed as pixel number. To quantify arterial endothelium, RECA-stained area (in red) was measured and expressed as a ratio (in percentage) to the phalloidin-stained area. To quantify nNOS expression, nNOS-stained dots (in red) were manually counted. To quantify cavernous smooth muscle, phalloidin-stained area (in green) was measured and expressed as a percentage of the entire corpus cavernosa.
Statistical analysis
Data were analyzed using Prism 5 (GraphPad Software, San Diego, CA) and expressed as mean ± standard error of mean (SEM). Multiple groups were compared using one-way analysis of variance followed by the Tukey-Kramer test for post-hoc comparisons. Statistical significance was set at p < 0.05.
Results
LESWT improves erectile function in diabetic rats
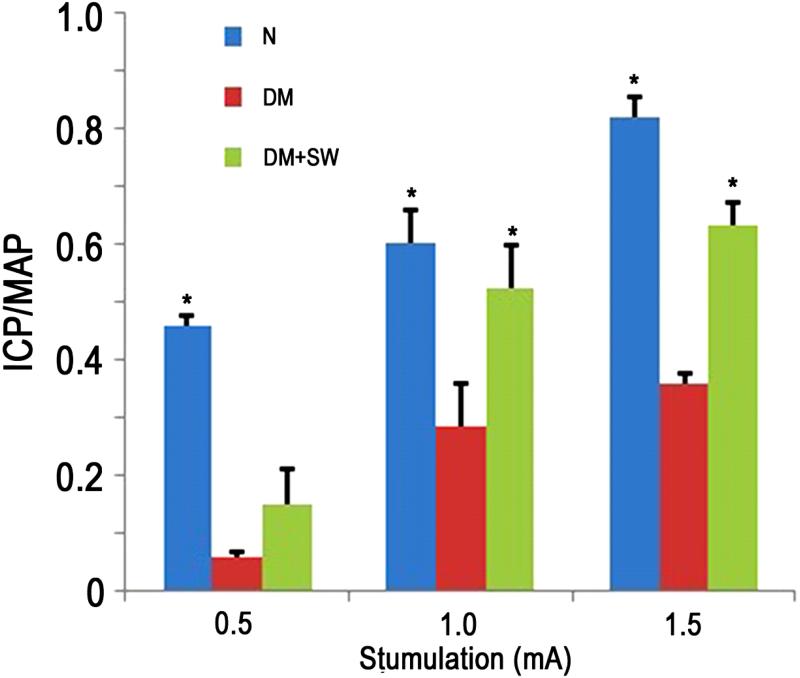
STZ treatment significantly impaired erectile function as seen in the sharp decline of the ICP/MAP value in DM rats versus normal control (Fig. 2). LESWT significantly restored erectile function to levels similar to normal control (at settings of 1.0 and 1.5 mA, Fig. 2).
Figure 2. Evaluation of erectile function.
Rats in N group (n=8) were normal control. Rats in DM group (n=8) were diabetic. Rats in DM+SW group (n=8) were diabetic and treated with shockwaves. Their erectile function was evaluated as response in ICP to electrostimulation of cavernous nerves at three different amperages (0.5, 1.0, and 1.5). ICP was normalized with MAP. * denotes p<0.05 when compared to the DM group.
LESWT promotes nerve regeneration
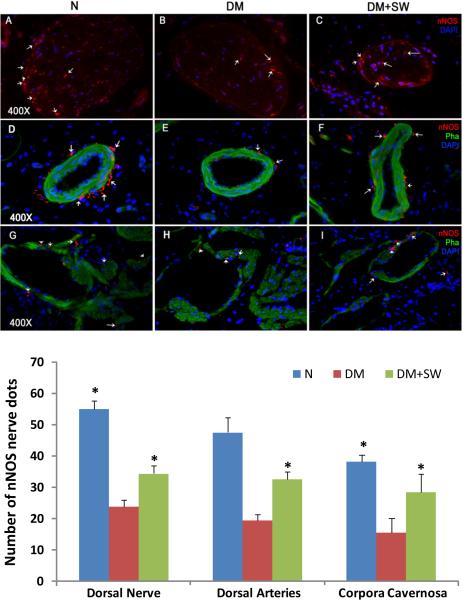
STZ treatment caused significant decreases of nNOS-containing nerves in the penis (Fig. 3). LESWT partially but significantly restored these nNOS-positive nerves in the sinusoids, around the dorsal arteries, and within the dorsal nerves (Fig. 3).
Figure 3. Evaluation of nNOS expression.
Rats were grouped and treated as described in Fig. 1. Their penile tissues were examined by IF staining for nNOS expression. The results are shown in the representative histological images with red, green, and blue stains indicating nNOS-positive nerves, smooth muscle, and cell nuclei, respectively. For clarity, the histological images are divided into the dorsal nerves (panels A-C), the dorsal arteries (panels D-F), and the sinusoids (panels G-I). White arrows point at representative nNOS-positive dots. Quantitative data of nNOS expression in these three tissue compartments are shown in the bar chart with the asterisk denoting p<0.05 when compared to the DM group.
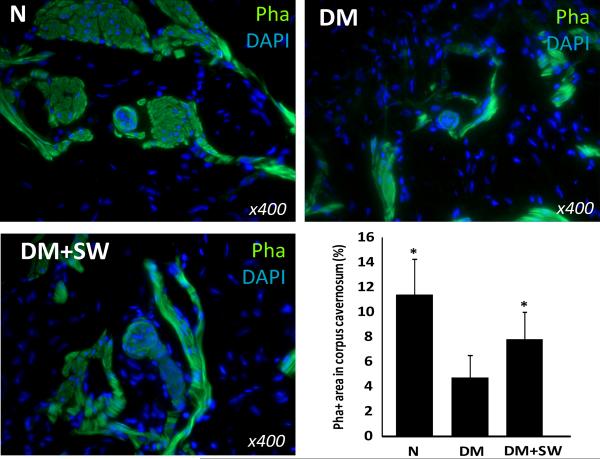
LESWT restores endothelial and smooth muscle contents
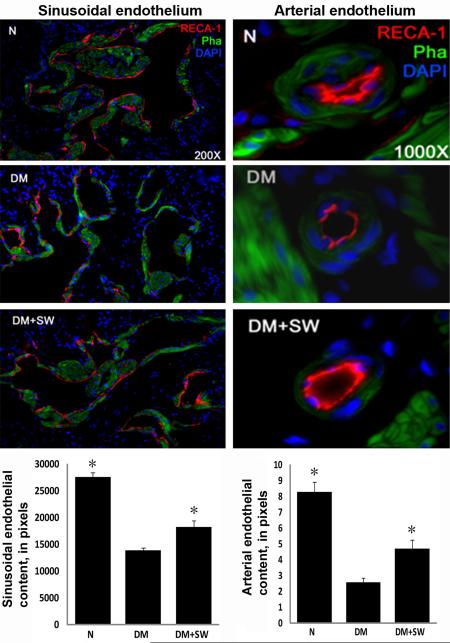
STZ treatment caused a significant decrease of endothelial content in both the cavernous sinusoids and small arteries, and which was partially but significantly reversed by LESWT (Fig. 4). Likewise, STZ treatment caused a significant reduction of cavernous smooth muscle content, and which was partially but significantly reversed by LESWT (Fig. 5).
Figure 4. Evaluation of endothelial content.
Rats were grouped and treated as described in Fig. 1. Their penile tissues were examined by IF staining for RECA expression. The results are shown in the representative histological images with red, green, and blue stains indicating the endothelium, smooth muscle, and cell nuclei, respectively. Quantitative data of RECA expression in cavernous sinusoids and arteries are shown in the left and right bar charts, respectively, with the asterisk denoting p<0.05 when compared to the DM group.
Figure 5. Evaluation of smooth muscle content.
Rats were grouped and treated as described in Fig. 1. Their penile tissues were examined by fluorescent phalloidin staining for smooth muscle. The results are shown in the representative histological images with green and blue stains indicating the smooth muscle and cell nuclei, respectively. Quantitative data of cavernous smooth muscle content are shown in the bar chart with the asterisk denoting p<0.05 when compared to the DM group.
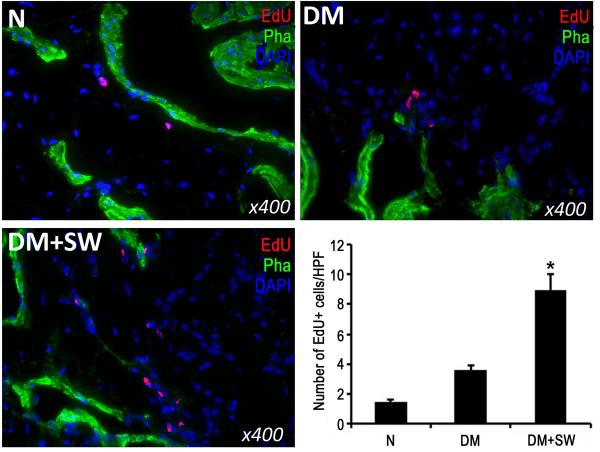
LESWT enhances recruitment of MSCs
MSCs, recognized by their ability to retain thymidine analog EdU, were significantly more numerous in the penis of rats in the DM+SW group than in the DM group (Fig. 6).
Figure 6. Evaluation of label-retaining cells.
Rats were intraperitoneally injected with thymidine analog EdU immediately after birth. They were then grouped and treated as described in Fig. 1. At 14 weeks post-EdU injection, their penile tissues were examined by fluorescent chemical staining for EdU+ cells. The results are shown in the representative histological images with red, green, and blue stains indicating EdU+ cells, smooth muscle, and cell nuclei, respectively. Quantitative data of EdU+ cells are shown in the bar chart with the asterisk denoting p<0.05 when compared to the DM group. HPF: high power field (400x).
Discussion
Despite tremendous advances in the management of ED in the past decade, DM-associated ED remains difficult to treat. To overcome this obstacle, one of the proposed therapeutic strategies is stem cell therapy, which has been actively pursued in several clinical and preclinical trials [16]. Another lesser-known strategy is LESWT, which has been tested in clinical trials, but in sharp contrast to stem cell therapy, has not been investigated at the preclinical level. Thus, the present study was designed to provide, for the first time, a mechanistic basis for LESWT’s therapeutic effects by using a well-established STZ-induced DM-ED rat model.
STZ-induced diabetic rats have been consistently shown to have poor erectile function [21, 22]. In the present study we further confirmed this observation, and more importantly, we showed that LESWT significantly improved erectile function in STZ-induced diabetic rats. It has also been known that STZ treatment caused a significant loss of nNOS-positive nerves in rat penis [22, 23], and a recent study also identified a significant reduction of nNOS-positive nerves in the penis of diabetic patients [24]. In the present study we showed that, when compared to DM rats, shockwave-treated rats displayed significantly higher numbers of nNOS-positive nerves in different compartments of the erectile tissue, including the dorsal nerves, around the dorsal arteries, and in the corpora cavernosa. This preservation of nNOS-positive nerves thus appears to be an underlying mechanism for LESWT’s therapeutic effects on diabetic patients.
Endothelial injury and dysfunction in cavernous tissue have been consistently identified in diabetic men with ED and in diabetic animal models [25]. Specifically, a reduced cavernous endothelial content is one the most consistent features of STZ-induced diabetic rats [22, 26]. In the present study we found that the endothelial contents in both the cavernous sinusoids and arteries were significantly reduced in STZ-treated rats. More importantly, we also found that LESWT was able to significantly restore the endothelial contents in both of these two tissue compartments. Thus, protection or regeneration of the endothelium represents another possible underlying mechanism for LESWT’s therapeutic efficacy in diabetic patients. In addition, it has also been shown that diabetic men and animals have reduced cavernous smooth muscle content [22, 26, 27]. In the present study we confirmed this finding in the STZ-treated rats, and more importantly, we showed that LESWT was able to significantly restore the smooth muscle content.
In all ED-related stem cell studies that have performed histological examination of the erectile tissue, restoration of nNOS-positive nerves, the endothelium, and the smooth muscle has also been consistently observed [16]. In addition, these studies also invariably pointed out that the beneficial tissue effects were likely mediated by stem cell’s paracrine capacity [16]. On the other hand, in non-ED fields, the involvement of stem cells in the therapeutic effects of LESWT has been observed in two instances. In one study of a rat model of bone defects, LESWT was found to result in the recruitment of MSCs and increased expression of TGF-β and VEGF in the defect tissues [11]. In another study of a rat model of chronic hind limb ischemia, LESWT was also found to enhance recruitment of endothelial progenitor cells in the ischemic tissue [10]. Thus, it is conceivable that the tissue effects of LESWT as observed in the present study might have a stem cell component.
LRC is a frequently employed strategy for the identification of resident or migrated stem cells in post-natal tissues [18-20]. It commonly involves the injection of thymidine analog BrdU in neonatal animals, followed by the immunohistochemical localization of BrdU in the tissue of interest weeks or months later. Because detection of BrdU is technically difficult, we recently introduced another thymidine analog EdU as a replacement for BrdU [18-20]. In the present study we repeated this strategy for the identification of migrated stem cells in the rat penis. The results show that diabetic rats with LESWT had a significantly higher number of EdU+ cells in the penis than diabetic rats without LESWT, suggesting an increased recruitment of MSCs.
In summary, the present study showed that STZ-induced DM is associated with ED and reduced erectile components (nerves, endothelium, and smooth muscle), and LESWT was able to partially but significantly restore these function and tissues. Furthermore, we also showed that these beneficial effects of LESWT were possibly mediated by increased recruitment of MSCs into the erectile tissue. However, it should be cautioned that the present study is still preliminary and requires further validation. Specifically, at 4 weeks after STZ treatment, the nitrergic degeneration has not yet taken place [23], but in clinical situations most patients already have nitrergic degeneration and hence no response to PDE5 inhibitors. Thus, in future studies extended time points should be explored in order to better simulate the clinical situation. In addition, the effect of LESWT on the vascular supply to the penis and major pelvic ganglion should be further investigated; and Western blot analysis should be employed to more accurately quantify the nNOS, endothelial and smooth muscle contents. Finally, the identity and properties of the EdU+ cells (paracrine? differentiation?) also need to be investigated.
Conclusions
LESWT’s therapeutic efficacy for DM-associated ED is possibly mediated by increased recruitment of MSCs that promote the regeneration of DM-damaged erectile tissues.
ACKNOWLEDGEMENTS
This work was funded in part by NIDDK/NIH R37 DK045370.
Footnotes
Conflict of Interest: None
References
- 1.Litwin MS, Nied RJ, Dhanani N. Health-related quality of life in men with erectile dysfunction. J Gen Intern Med. 1998;13:159–66. doi: 10.1046/j.1525-1497.1998.00050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dorsey P, Keel C, Klavens M, Hellstrom WJ. Phosphodiesterase type 5 (PDE5) inhibitors for the treatment of erectile dysfunction. Expert Opin Pharmacother. 2010;11:1109–22. doi: 10.1517/14656561003698131. [DOI] [PubMed] [Google Scholar]
- 3.Gruenwald I, Appel B, Vardi Y. Low-intensity extracorporeal shock wave therapy--a novel effective treatment for erectile dysfunction in severe ED patients who respond poorly to PDE5 inhibitor therapy. J Sex Med. 2012;9:259–64. doi: 10.1111/j.1743-6109.2011.02498.x. [DOI] [PubMed] [Google Scholar]
- 4.Vardi Y, Appel B, Jacob G, Massarwi O, Gruenwald I. Can low-intensity extracorporeal shockwave therapy improve erectile function? A 6-month follow-up pilot study in patients with organic erectile dysfunction. Eur Urol. 2010;58:243–8. doi: 10.1016/j.eururo.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 5.Vardi Y, Appel B, Kilchevsky A, Gruenwald I. Does low intensity extracorporeal shock wave therapy have a physiological effect on erectile function? Short-term results of a randomized, double-blind, sham controlled study. J Urol. 2012;187:1769–75. doi: 10.1016/j.juro.2011.12.117. [DOI] [PubMed] [Google Scholar]
- 6.Zimpfer D, Aharinejad S, Holfeld J, et al. Direct epicardial shock wave therapy improves ventricular function and induces angiogenesis in ischemic heart failure. J Thorac Cardiovasc Surg. 2009;137:963–70. doi: 10.1016/j.jtcvs.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 7.Fu M, Sun CK, Lin YC, et al. Extracorporeal shock wave therapy reverses ischemia-related left ventricular dysfunction and remodeling: molecular-cellular and functional assessment. PLoS One. 2011;6:e24342. doi: 10.1371/journal.pone.0024342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nishida T, Shimokawa H, Oi K, et al. Extracorporeal cardiac shock wave therapy markedly ameliorates ischemia-induced myocardial dysfunction in pigs in vivo. Circulation. 2004;110:3055–61. doi: 10.1161/01.CIR.0000148849.51177.97. [DOI] [PubMed] [Google Scholar]
- 9.Mittermayr R, Hartinger J, Antonic V, et al. Extracorporeal shock wave therapy (ESWT) minimizes ischemic tissue necrosis irrespective of application time and promotes tissue revascularization by stimulating angiogenesis. Ann Surg. 2011;253:1024–32. doi: 10.1097/SLA.0b013e3182121d6e. [DOI] [PubMed] [Google Scholar]
- 10.Aicher A, Heeschen C, Sasaki K, Urbich C, Zeiher AM, Dimmeler S. Low-energy shock wave for enhancing recruitment of endothelial progenitor cells: a new modality to increase efficacy of cell therapy in chronic hind limb ischemia. Circulation. 2006;114:2823–30. doi: 10.1161/CIRCULATIONAHA.106.628623. [DOI] [PubMed] [Google Scholar]
- 11.Chen YJ, Wurtz T, Wang CJ, et al. Recruitment of mesenchymal stem cells and expression of TGF-beta 1 and VEGF in the early stage of shock wave-promoted bone regeneration of segmental defect in rats. J Orthop Res. 2004;22:526–34. doi: 10.1016/j.orthres.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Lee MC, El-Sakka AI, Graziottin TM, Ho HC, Lin CS, Lue TF. The effect of vascular endothelial growth factor on a rat model of traumatic arteriogenic erectile dysfunction. J Urol. 2002;167:761–7. doi: 10.1016/S0022-5347(01)69141-9. [DOI] [PubMed] [Google Scholar]
- 13.Lin CS, Ho HC, Chen KC, Lin G, Nunes L, Lue TF. Intracavernosal injection of vascular endothelial growth factor induces nitric oxide synthase isoforms. BJU Int. 2002;89:955–60. doi: 10.1046/j.1464-410x.2002.02792.x. [DOI] [PubMed] [Google Scholar]
- 14.Lin CS, Lue TF. Growth factor therapy and neuronal nitric oxide synthase. Int J Impot Res. 2004;16(Suppl 1):S38–9. doi: 10.1038/sj.ijir.3901214. [DOI] [PubMed] [Google Scholar]
- 15.Lin CS, Xin ZC, Deng CH, Ning H, Lin G, Lue TF. Recent advances in andrology-related stem cell research. Asian J Androl. 2008;10:171–5. doi: 10.1111/j.1745-7262.2008.00389.x. [DOI] [PubMed] [Google Scholar]
- 16.Lin CS, Xin ZC, Wang Z, et al. Stem cell therapy for erectile dysfunction: a critical review. Stem Cells Dev. 2012;21:343–51. doi: 10.1089/scd.2011.0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bickenbach JR, Chism E. Selection and extended growth of murine epidermal stem cells in culture. Exp Cell Res. 1998;244:184–95. doi: 10.1006/excr.1998.4163. [DOI] [PubMed] [Google Scholar]
- 18.Lin G, Huang YC, Shindel AW, et al. Labeling and tracking of mesenchymal stromal cells with EdU. Cytotherapy. 2009;11:864–73. doi: 10.3109/14653240903180084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin G, Xin Z, Zhang H, et al. Identification of active and quiescent adipose vascular stromal cells. Cytotherapy. 2012;14:240–6. doi: 10.3109/14653249.2011.627918. [DOI] [PubMed] [Google Scholar]
- 20.Zhang H, Lin G, Qiu X, et al. Label retaining and stem cell marker expression in the developing rat urinary bladder. Urology. 2012;79:746, e1–6. doi: 10.1016/j.urology.2011.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qiu X, Lin H, Wang Y, et al. Intracavernous transplantation of bone marrow-derived mesenchymal stem cells restores erectile function of streptozocin-induced diabetic rats. J Sex Med. 2011;8:427–36. doi: 10.1111/j.1743-6109.2010.02118.x. [DOI] [PubMed] [Google Scholar]
- 22.Zhou F, Xin H, Liu T, et al. Effects of Icariside II on Improving Erectile Function in Rats With Streptozotocin-Induced Diabetes. Journal of andrology. 2012;33:832–44. doi: 10.2164/jandrol.111.015172. [DOI] [PubMed] [Google Scholar]
- 23.Cellek S, Foxwell NA, Moncada S. Two phases of nitrergic neuropathy in streptozotocin-induced diabetic rats. Diabetes. 2003;52:2353–62. doi: 10.2337/diabetes.52.9.2353. [DOI] [PubMed] [Google Scholar]
- 24.Dashwood MR, Crump A, Shi-Wen X, Loesch A. Identification of neuronal nitric oxide synthase (nNOS) in human penis: a potential role of reduced neuronally-derived nitric oxide in erectile dysfunction. Current pharmaceutical biotechnology. 2011;12:1316–21. doi: 10.2174/138920111798280965. [DOI] [PubMed] [Google Scholar]
- 25.Thorve VS, Kshirsagar AD, Vyawahare NS, Joshi VS, Ingale KG, Mohite RJ. Diabetes-induced erectile dysfunction: epidemiology, pathophysiology and management. J Diabetes Complications. 2011;25:129–36. doi: 10.1016/j.jdiacomp.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Albersen M, Lin G, Fandel TM, et al. Functional, Metabolic, and Morphologic Characteristics of a Novel Rat Model of Type 2 Diabetes-associated Erectile Dysfunction. Urology. 2011;78:476, e1–8. doi: 10.1016/j.urology.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gratzke C, Angulo J, Chitaley K, et al. Anatomy, physiology, and pathophysiology of erectile dysfunction. J Sex Med. 2010;7:445–75. doi: 10.1111/j.1743-6109.2009.01624.x. [DOI] [PubMed] [Google Scholar]