Figure 1. MRM transitions and quantitative analysis of endogenous human mitochondrial phosphorylations.
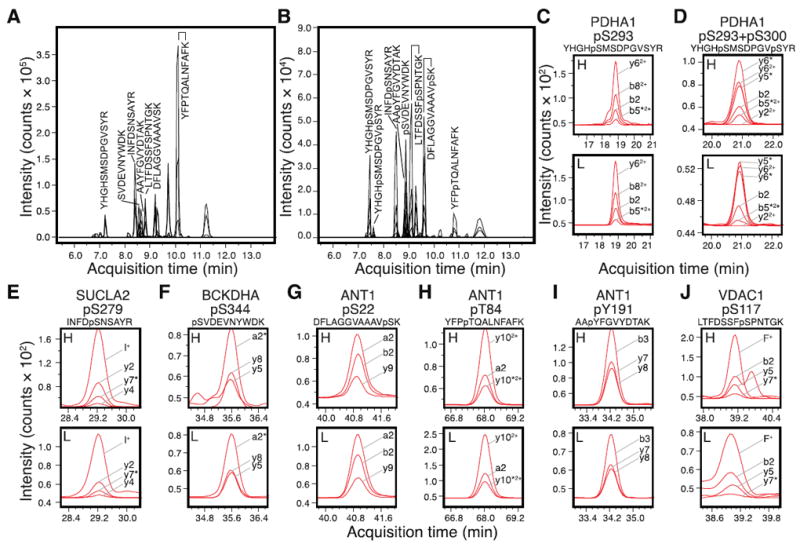
The new set of MRM assays were validated using heavy-isotope-labeled synthetic peptide standards of (A) unmodified peptides and (B) their respective phosphorylated versions. The peaks corresponding to peptides from PDH, VDAC, ANT, shown in C-J, are labeled with their sequences in the chromatogram. (C-J) Human protein phosphorylation was detected by comparing the heavy-labeled standard (H) and unlabeled endogenous peptides (L). The MRM signals for a panel of transitions are shown and labeled; asterisks next to the fragment ions denote neutral loss of H3PO4. (C) Pyruvate dehydrgeonase E1 complex alpha subunit (PDHA1) pS293 phosphopeptide YHGHpSMSDPGVSYR. (D) PDHA1 pS293+pS300 doubly-phosphorylated peptide YHGHpSMSDPGVpSYR. (E) Succinyl-CoA ligase [ADP-forming] subunit beta (SUCLA2) pS279 phosphopeptide INFDpSNSAYR. (F) Branched-chain alpha-keto acid dehydrogenase subunit alpha (BCKDHA) pS344 phosphopeptide pSVDEVNYWDK. (G) Adenine nucleotide translocase 1 (ANT1) pS22 phosphopeptide DFLAGGVAAAVpSK. (H) ANT1 pT84 phosphopeptide YFPpTQALNFAFK. (I) ANT1 pY191 phosphopeptide AApYFGVYDTAK. (J) Voltage-dependent anion channel I (VDAC1) pS117 phosphopeptide LTFDSSFpSPNTGK. For consistency, amino acid residue numbering is based on mouse homolog sequence in Uniprot [19].
