Figure 5.
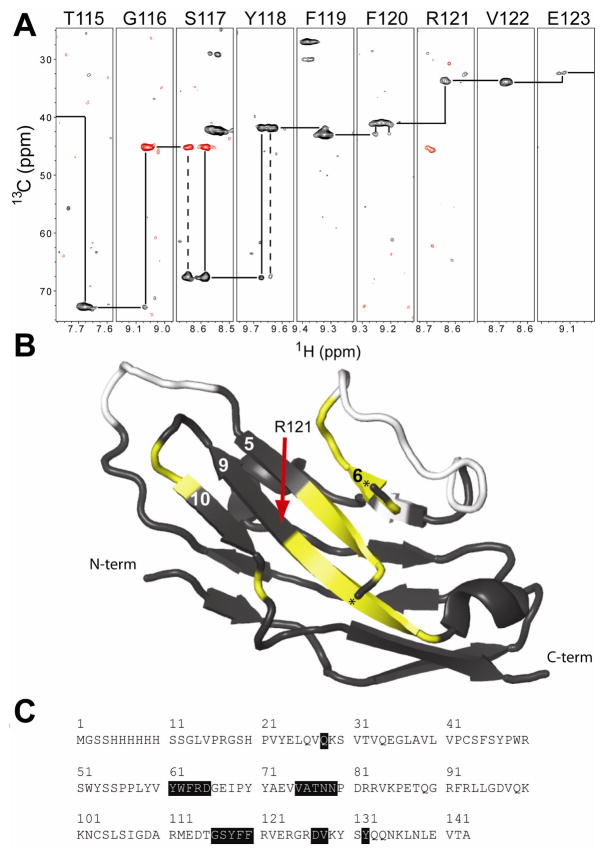
NMR experiments indicated alternate forms of Siglec5. (A) A sequence walk of an HN(CA)CB experiment showed alternative conformations and weak resonances on the beta strand formed by residues 115–123. Positive (negative) signals are shown with black (red) contours. (B) Such patterns were observed along many portions of the protein sequence. These are shown in yellow mapped on the black ribbon diagram of Siglec5 and in (C). Unassigned residues are shown in white. A small helix obscured the view of the central beta sheet and is not shown; the termini of this helix are marked with a “★.”