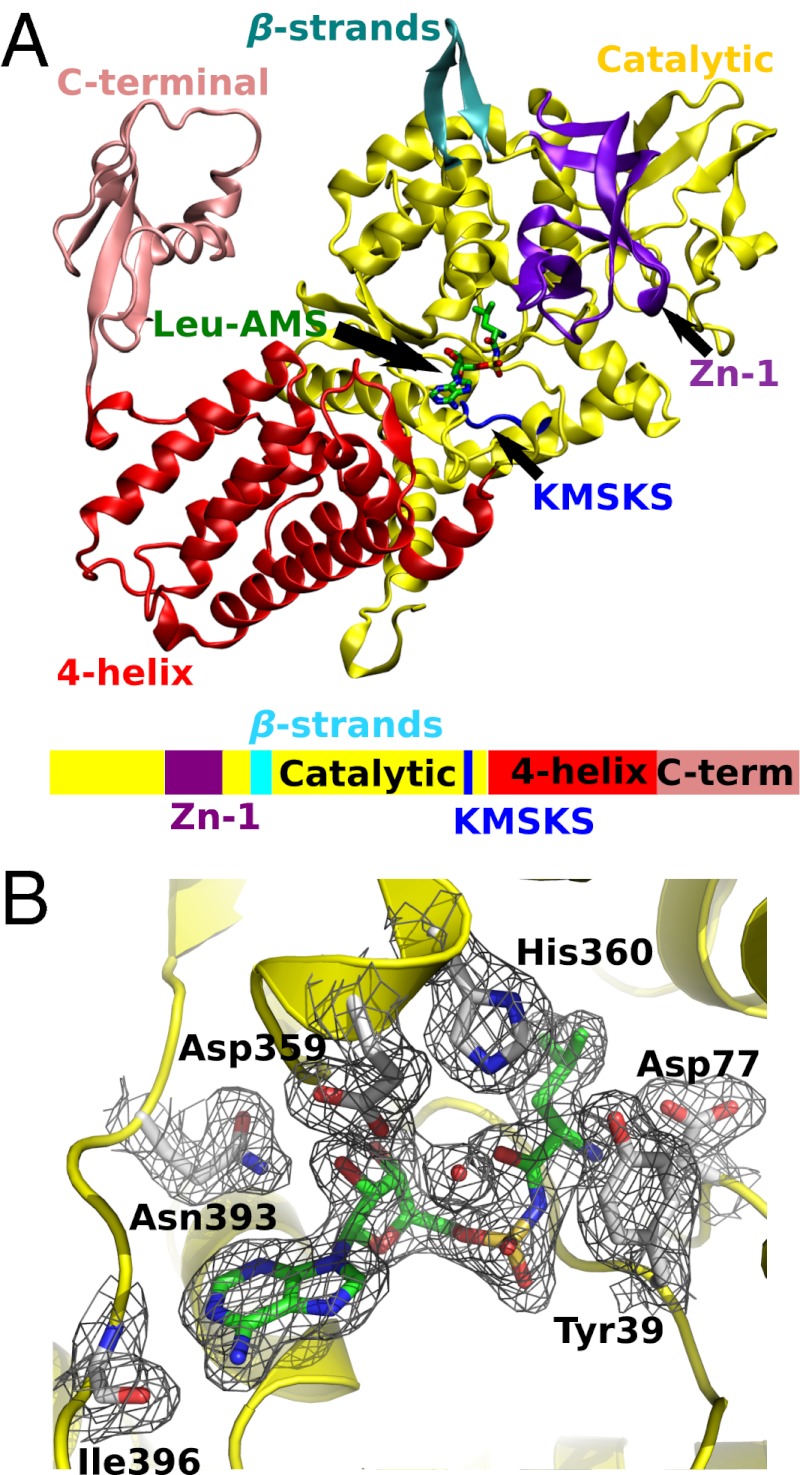
Fig. 3.
X-ray crystal structure of M. mobile LeuRS Leu-AMS complex. (A) A ribbon diagram of the protomer with electron density of C-terminal domain is colored as follows: catalytic domain, yellow; four-helix bundle domain, red; C-terminal domain, pink; Zn binding domain, purple; KMSKS loop, blue; two linking β-strands, cyan. The Leu-AMP analog, Leu-AMS, is shown in stick model. A color-coordinated cartoon of the primary sequence is shown below the structure. (B) The 2Fo-Fc electron density map of the aminoacylation active site is contoured at 1.0 σ (black mesh). The Leu-AMS is highlighted in green, and interacting amino acid residues are labeled and shown in gray.