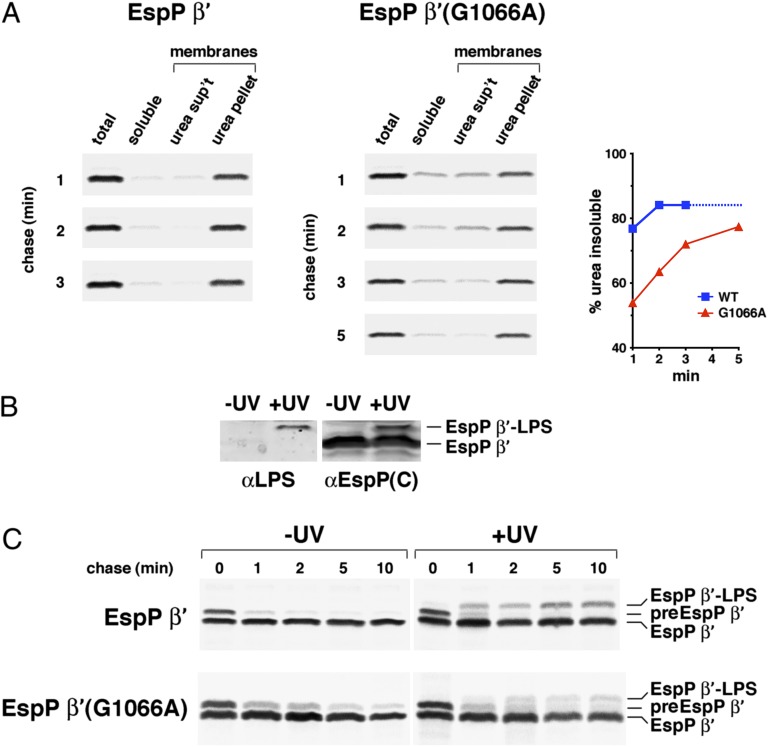
Fig. 7.
The G1066A mutation delays the membrane integration of the EspP β domain. (A) AD202 transformed with pJH61 (Ptrc-espP β′) or a pJH61 derivative encoding EspP β′(G1066A) were subjected to pulse-chase labeling after the addition of IPTG. Cells were fractionated and equivalent amounts of each fraction were used for immunoprecipitations with an anti-EspP C-terminal antiserum. The percent of the protein recovered during the fractionation procedure that was isolated in the membrane fraction and that was resistant to urea extraction at each time point is shown. We assume that the percent of wild-type EspP β′ that was urea insoluble did not increase after 3 min (dotted line). (B) AD202 transformed with pDULEBpa and pJH61 were incubated with IPTG. Cell membrane fractions were then analyzed by Western blot using anti-LPS and anti-EspP C-terminal antisera. (C) AD202 transformed with pDULEBpa and pJH61 or a pJH61derivative encoding EspP β′(G1066A) were subjected to pulse-chase labeling after the addition of IPTG. One aliquot of cells was UV-irradiated and an equal aliquot was untreated, and immunoprecipitations were conducted using an anti-EspP C-terminal antiserum.