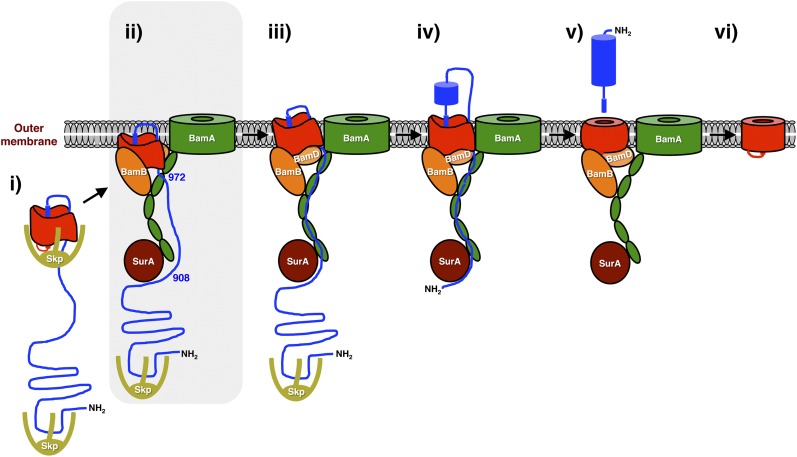
Fig. 8.
Model of EspP biogenesis. The EspP β domain undergoes considerable folding in the periplasm and incorporates the C terminus of the passenger domain in a hairpin configuration (i). The protein is then targeted to the OM, where specific regions of the β domain interact with BamA, BamB, and BamD. Previously unpublished results presented here indicate that the hairpin is not exposed on the cell surface at this stage and suggest that although the passenger domain is not bound stably to the BamA periplasmic (POTRA) domains, residues 908 and 972 are in close proximity to SurA and BamA, respectively (ii, shaded). A conformational change in the β domain is subsequently required to “license” the initiation of passenger domain translocation (iii). The passenger domain then interacts with the BamA POTRA domains and moves in a stepwise or processive fashion to a transport channel composed of the β domain, BamA and possibly other proteins. After the initiation of translocation (iv), the β domain undergoes an additional conformational change. Following the completion of translocation, the passenger domain is released in an intrabarrel cleavage reaction (v). Finally, the β domain is released into the lipid bilayer following the completion of its assembly (vi). Many aspects of this model are based on previous studies. For simplicity, BamC and BamE have been omitted. BamD is also largely hidden in step ii.