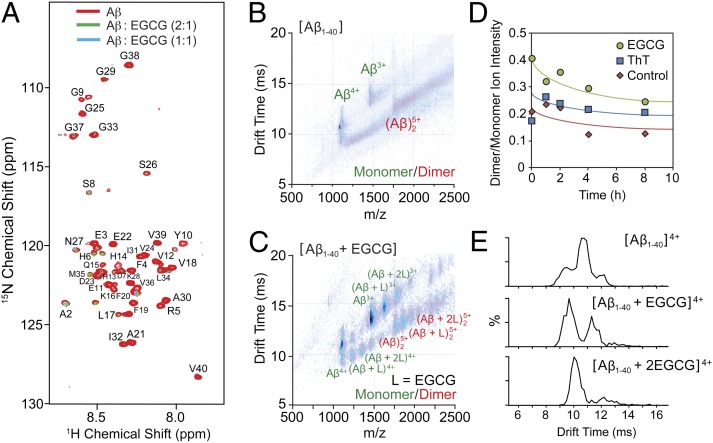
Fig. 2.
Interaction of EGCG with Aβ1–40. (A) Titration of Aβ with EGCG monitored by 2D 1H/15N SOFAST-HMQC NMR spectroscopy. Substoichiometric amounts of EGCG caused a large uniform decrease in intensity. (B) A plot of IM drift time vs. m/z for apo-Aβ1–40 (10 μM) showed monomeric and dimeric forms of the peptide under conditions used for our experiments (100 mM ammonium acetate buffer, pH 7.4). (C) Similar IM-MS data as in B for samples containing EGCG added in solution (20 µM) revealed multiple binding modes for EGCG with the monomeric and dimeric form of Aβ. (D) MS-based time course experiments presented that EGCG (green circles) could solubilize larger amounts of the dimeric forms of the peptide in solution compared with ThT (blue squares) and only peptide (red diamonds; control). (E) Close inspection of the IM drift time profiles for the 4+ state of the peptide indicated at least three resolved conformational families and that EGCG binding could produce a larger population of compact Aβ1–40 peptides (CCS: 796, 716, and 633 Å2, Top to Bottom; Table S1).