Fig. 4.

Removal of the conserved H120–H43–T39 linkage by mutation H43F. (A) X-ray structures of apoSOD1ΔIVΔVII (PDB entry 4BCZ) and apo (PDB entry 4BD4) show that H43F leads to relaxation of the twisted backbone carbonyl of H120 into the sheet where it H-bonds to the amide of G44. (B) Cu2+ coordination twists back the carbonyl of H120 into the core, forcing the protein into a native-like metal-binding geometry. Holo
(PDB entry 4BD4) show that H43F leads to relaxation of the twisted backbone carbonyl of H120 into the sheet where it H-bonds to the amide of G44. (B) Cu2+ coordination twists back the carbonyl of H120 into the core, forcing the protein into a native-like metal-binding geometry. Holo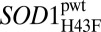 (PDB entry 4BCZ).
(PDB entry 4BCZ).
