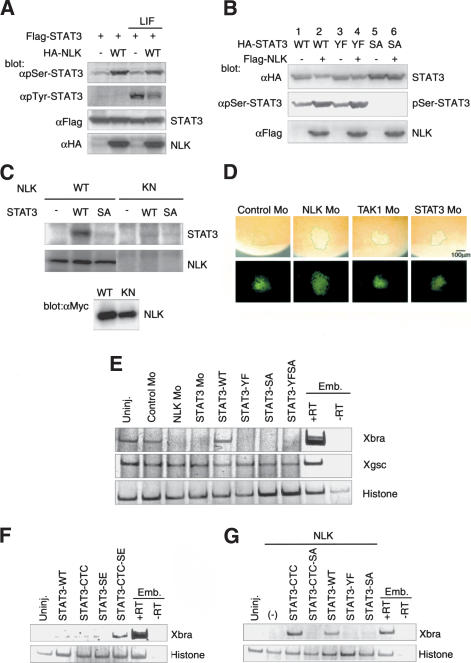
Figure 3.
NLK phosphorylates the C-terminal serine residue of STAT3. (A,B) 293 cells were transfected as indicated and treated with LIF or left untreated, and whole-cell lysates were subjected to Western blotting by using the indicated antibodies. (C) 293 cells were transfected with Myc-tagged wild-type NLK (WT) or kinase-negative NLK (KN). Immunoprecipitates obtained with anti-Myc antibody were incubated with bacterially expressed STAT3 and [γ-32P] ATP. Phosphorylation of STAT3 (top) and autophosphorylation of NLK (middle) are shown. Expression of Myc-NLK was monitored (bottom). (D) The indicated Mo and lineage tracer FLDx (green; bottom) were injected into the marginal zone of 32-cell-stage embryos. Whole-mount immunostaining with anti-pSer-STAT3 antibody was performed at stage 11 (top). Green lines indicate the boundary of the injected area. (E) RT–PCR analysis of the expression of mesodermal markers in DMZ. Control Mo (10 ng), NLK Mo (8.4 ng), STAT3 Mo (10 ng), or synthetic mRNA of wild-type STAT3 or its mutants (2 ng) were injected as indicated. (F,G) RT–PCR analysis of expression of Xbra in animal caps. (F) Wild-type STAT3 or mutant mRNAs (5 ng) were injected as indicated. (G) NLK mRNA (2.5 ng) was injected with or without STAT3 mutant mRNAs (2.5 ng) as indicated.