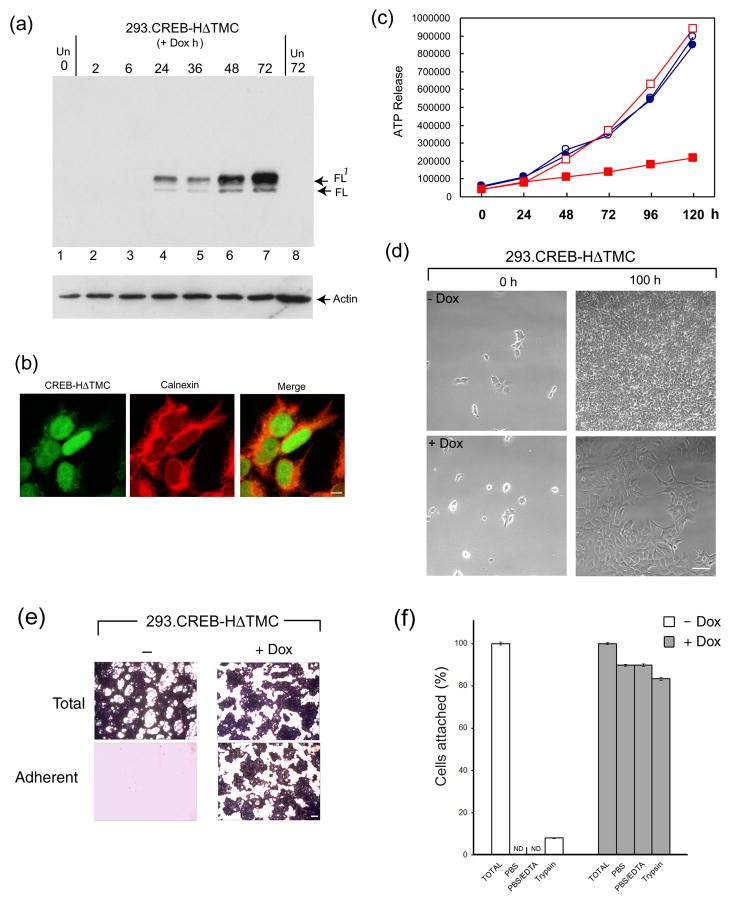
Figure 3. Establishment of HEK-293 cells with regulated expression of CREB-HΔTMC.
(a) HEK-293 cells clonally selected for doxycycline regulated expression of CREB-HΔTMC were harvested at different times after induction (0.5 μg/ml of doxycycline) or after control treatment (Un). Tightly regulated expression of CREB-HΔTMC can be seen, from undetectable in untreated cells, increasing between 6 and 24 h after Dox addition. The doublet represents full length CREB-HΔTMC (FL) and a phosphorylated species FL1 as previously demonstrated (22). (b) Induced cells were fixed and stained for the detection of CREB-HΔTMC (green), which localized primarily in the nucleus. Cells were counterstained with the ER marker calnexin (red). Scale bar, 10 μM. (c) 293.CREB-HΔTMC cells ( Dox, open squares: +Dox, filled squares) or a control 293 cell line (filled circles: Dox, open circles: +Dox, filled squares) were seeded at low and incubated in standard growth medium. Dox was added to a duplicate series of each cell line 24 h after seeding. Cells were harvested at intervals thereafter and cell proliferation measured using the Promega luminescent cell viability assay. (d) Phase images of 293.CREB-HΔTMC cells immediately after plating (0 h) or after approximately 4 days (100 h) in the absence or presence of Dox. Scale bar, 100 μM (e) CREB-H induces increased cell adhesion. 293.CREB-HΔTMC cells in cluster dishes (105 cells per well), were induced with Dox or mock treated, incubated for 5 days, then fixed with paraformaldehyde and stained with crystal violet (Total). Duplicate cultures were aspirated, washed with PBS, then fixed and stained (Adherent). Scale bar 1 mm (f) Quantification of cell adhesion as described in the text. Open bars represent cultures grown in the absence of Dox, filled bars represent plus Dox. The total cell recovery was set to 100% in each case. Adherent cells numbers were below the level of significant detection (ND) in the uninduced cultures. The values are averages of three independent experiments, error bars correspond to standard deviations.