Figure 2.
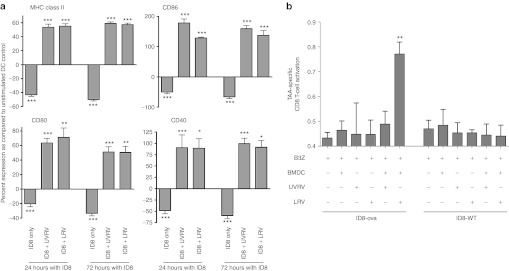
Overcoming OC-induced dysfunction of DCs. (a) ID8 cells and BMDCs were co-cultured together for 24 or 72 hours before infection with 10 MOI of reovirus for 48 hours. Cells were stained with antibodies against CD11c and either MHC II, CD86, CD80, and CD40 and analyzed using flow cytometry. Bars show respective percentages normalized against, and asterisks show statistical analysis as compared with, BMDCs cultured without ID8 or reovirus. The data is representative of at least five independent experiments. (b) 2 × 105 BMDCs were co-cultured with 2 × 105 ID8-ova cells and added with LRV/UVRV for 24 hours. Next, co-cultures were washed, added with 1 × 105 B3Z cells per well, incubated for additional 18–24 hours and then added with 0.15 mmol/l of CPRG for additional 4 hours. The breakdown of GPRG was read at 570 nm as a measure of CD8+ T-cell response. Statistical analysis was obtained by comparing readings from each experimental condition against that observed in only the B3Z wells; ns = P > 0.05; *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001. The data is cumulative from three independent experiments. BMDC, bone marrow-derived DCs; CPRG, chlorophenol red-β-D-galactopyranoside; DC, dendritic cell; MHC, major histocompatibility complex; MOI, multiplicity of infection; ns, not significant; LRV, live reovirus; TAA, tumor-associated antigen; UVRV, UV-inactivated reovirus.
