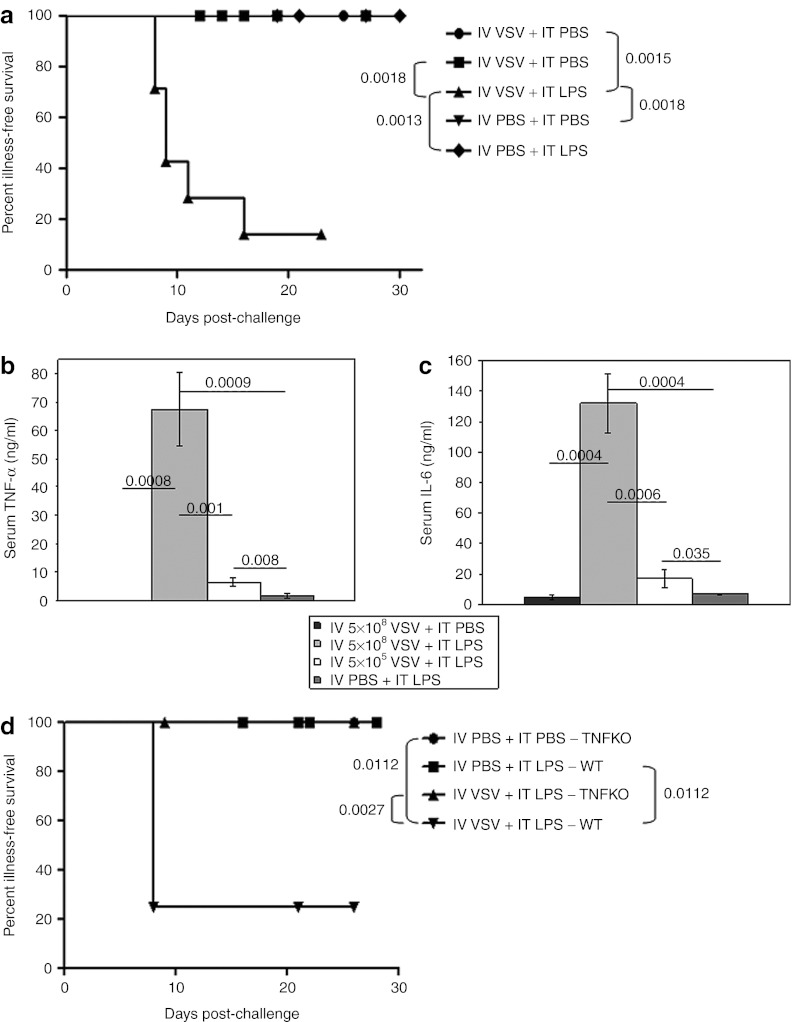
Figure 4.
High-dose, systemic VSV, when combined with local LPS treatment, results in acute toxicity. (a) Illness-free survival of C57BL/6 mice (mice not showing any signs of physical distress) bearing subcutaneous tumors after treatment with intravenous (IV) VSV-Δ51 (5 × 108 PFU/100 µl) or PBS (100 µl) followed by intratumoral (IT) PBS (50 µl) or LPS (200 µg/50 µl) 6 hours later (n = 7/group). Injections started on day 7 and continued every other day for a total of four (days 7, 9, 11, 13). (b,c) Mice (as in a) were treated with IV VSV-Δ51 (5 × 108 PFU/100 µl or 5 × 105 PFU/100 µl) or PBS (100 µl) followed by IT PBS (50 µl) or LPS (200 µg/50 µl) 6 hours later (n = 7/group). Cardiac terminal bleeds were done 1.5 hours after a single combination treatment. Serum (b) TNF-α or (c) IL-6 levels were determined by ELISA and are shown as mean ± SD. (d) Illness-free survival (as in a) of tumor-bearing C57BL/6 or TNFKO mice after a single treatment with IV VSV-Δ51 (5 × 108 PFU/100 µl) or PBS (100 µl) followed by IT PBS (50 µl) or LPS (200 µg/50 µl) 6 hours later (LPS only groups: n = 5/group; VSV + LPS groups: n = 8/group). ELISA, enzyme-linked immunosorbent assay; IL, interleukin; KO, knockout; LPS, lipopolysaccharide; PBS, phosphate-buffered saline; PFU, plaque-forming unit; TNF-α, tumor necrosis factor-α VSV, vesicular stomatitis virus; WT, wild-type.