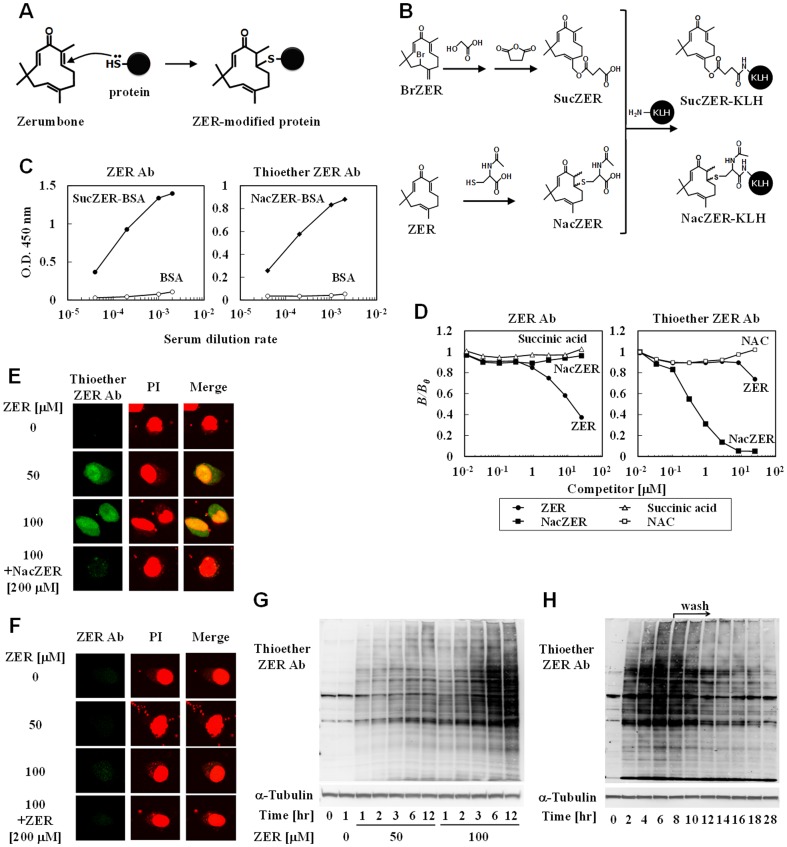
Figure 1. ZER non-selectively reacted with cellular proteins.
(A) Nucleophilic addition of a protein thiol to zerumbone (ZER). (B) ZER was chemically derivatized to SucZER (succinylated ZER derivative) and NacZER (NAC-modified ZER derivative), followed by conjugation with KLH by carbodiimide procedure to generate Abs against ZER and its thiol adducts. (C) Generated polyclonal Ab (ZER Ab and thioether ZER Ab) titers were determined by ELISA, with each hapten moiety conjugated with BSA (SucZER-BSA and NacZER-BSA) or native BSA. These experiments were performed in duplicates. (D) Cross-reactivities of the Abs were determined by competitive ELISA. Competitors such as ZER, NacZER, succinic acid, and NAC (N-acetyl-L-cysteine) were added to hapten moiety conjugated BSA-coated wells with a primary Ab solution. The cross-reactivity of the antibody to the competitors was expressed as B/B 0, in which B is the amount of antibody bound to the coating antigen in the presence of the competitor and B 0 that in the absence of a competitor. These experiments were performed in duplicates. (E, F) Hepa1c1c7 cells were treated with ZER (0, 50, 100 µM) for 30 minutes, then fixed with 4% paraformaldehyde and permeabilized with 0.1% Triton X-100, followed by immunostaining with thioether ZER Ab (E) or ZER Ab (F). NacZER (E) or ZER (F) was added with the primary Ab as a competitor. Images showing cellular immunofluorescence (green: FITC, red: PI) were acquired using a confocal laser scanning microscope (original magnification: ×400). (G) Cells were treated with ZER (0, 50, 100 µM) for 1 minute to 12 hours, then lysed for western blot analysis using thioether ZER Ab. (H) Cells were treated with ZER (50 µM) for 0–6 hours, then washed with PBS and incubation in BioZER-free DMEM for another 2–22 hours. Cells were lysed for detection of modified proteins by western blot analysis with thioether ZER Ab.