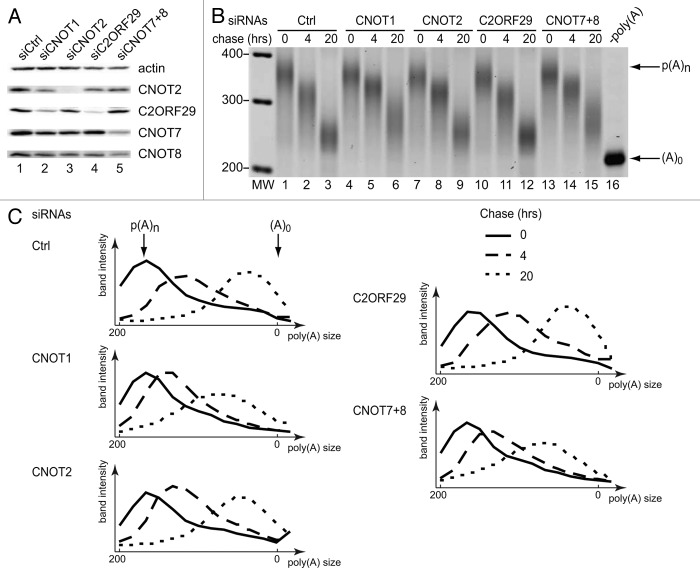
Figure 6. Depletion of C2ORF29 does not affect the deadenylation rate of a β-globin mRNA reporter. (A) Western Blot showing the depletion levels of the indicated proteins obtained after siRNA treatment. The depletion of CNOT1 could not be monitored by western blotting because no good quality antibodies were available. (B) Poly(A) tail shortening of the β-globin mRNA reporter. HEK293-TOF cells were first transfected with the indicated siRNAs and simultaneously re-transfected 24 h later with the siRNAs and a plasmid expressing the β-globin mRNA reporter. Immediately after transfection, doxycyclin (1 ng/ml) was added to the medium to block transcription of the reporter. Two days later, cells were washed and a 4 h transcriptional pulse was performed before re-addition of doxycyclin (2 μg/ml). Chase times indicate hours after the second doxycyclin addition. The poly(A) tail length of the reporter was analyzed by a RT-PCR based assay.36 An RNA sample treated with oligo(dT) and RNase H was used as a marker for the migration of the fully deadenylated β-globin mRNA[-poly(A)]. (C) Migration profiles illustrating the extent of deadenylation of the reporter transcript. Each lane of the gel presented in Figure 6B was divided in 14 identical rectangles and the signal intensity for each rectangle quantified with the ImageQuant software (GE Healthcare). This revealed that deadenylation rates in cells depleted of CNOT1, CNOT2, or CNOT7 and CNOT8 were reduced by roughly 50% compared with control cells or C2ORF29-depleted cells. A significant fraction of the residual activity may originate from other deadenylases (e.g., PAN2).

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.