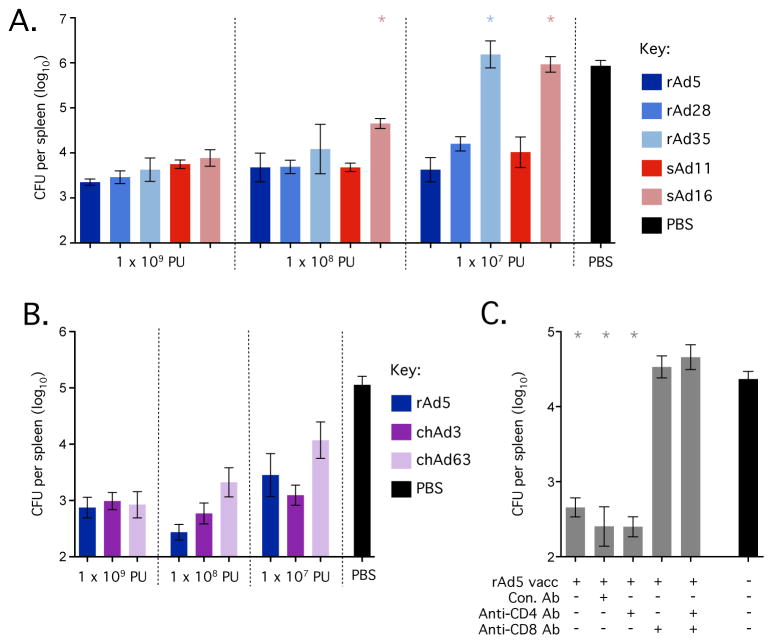
Figure 6.
Protection afforded by vaccination with rAd vectors against intravenous challenge with Listeria:Gag. (A) Bacterial load in the spleen (colony forming units, CFU) after challenge of mice vaccinated with the indicated doses of rAd5, rAd28, rAd35, sAd11 or sAd16. (B) Bacterial load in the spleen (CFU) after challenge of mice vaccinated with the indicated doses of rAd5, chAd3 or chAd63. (C) Bacterial load in the spleen (CFU) after challenge of mice vaccinated with 1 × 109 PU of rAd5 and either left untreated or treated with a control antibody (Con. Ab), a CD4-depleting antibody (Anti-CD4 Ab), a CD8-depleting Ab (Anti-CD8 Ab) or both of the latter. Each group contained 3–6 C57BL/6 mice and all challenges used a 2 × 107 CFU dose of Listeria:Gag administered intravenously. Significant differences in bacterial load were assessed for each vector compared to rAd5 at the equivalent dose (A/B) or compared to the naïve control (C), where * = p ≤ 0.05. Bars and error bars represent geometric mean ± GEM. Each group is representative of at least two independent experiments.