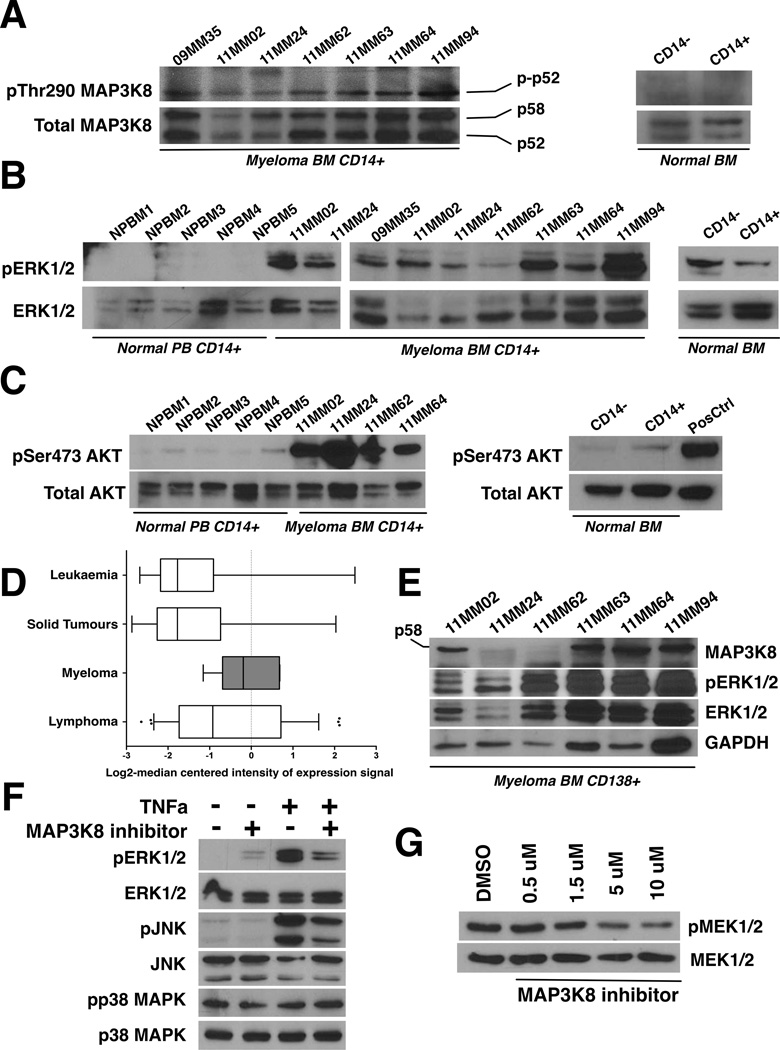
Figure 1.
A. MAP3K8 kinase is constitutively active in myeloma-associated monocytes/macrophages. Bone marrow (BM) aspirates were collected with informed consent from 7 patients (“MM” lanes). Fresh BM from normal donors was obtained commercially. CD14+ mononuclear cells were purified by immunomagnetic separation and lysates were immunoblotted using antisera against phosphorylated (phospho-Thr290) and total MAP3K8. For the normal bone marrow control, CD14+ denotes the CD14+ mononuclear cell fraction (positive isolation) and CD14- denotes the CD14-depleted mononuclear fraction. Two different normal marrow donors gave identical results. Both p58 and p52 MAP3K8 isoforms are expressed in CD14+ cells at comparable rates. The phosphorylated p58 isoform is prone to rapid degradation (Gantke, et al 2011).
B. Constitutive ERK activation in myeloma-associated monocytes/macrophages. The activation status of ERK was probed using antibodies against phosphorylated and total ERK. CD14+ peripheral blood monocytes from 5 different normal donors are shown to the far left (NPBM1-5) next to CD14+ bone marrow mononuclear cells from myeloma patients (“MM” lanes). For the normal bone marrow control, CD14+ denotes the CD14+ mononuclear cell fraction (positive isolation) and CD14- denotes the CD14-depleted mononuclear fraction. Two different normal marrow donors gave identical results.
C. Constitutive AKT activation at Ser473 in myeloma-associated monocytes/macrophages. The activation status of AKT was probed using antibodies against phosphorylated (phospho-Ser473 AKT) and total AKT. CD14+ peripheral blood monocytes from 5 different normal donors are shown to the far left (NPBM1-5) next to CD14+ bone marrow mononuclear cells from myeloma patients (“MM” lanes). For the normal bone marrow control, CD14+ denotes the CD14+ mononuclear cell fraction (positive isolation) and CD14- denotes the CD14-depleted mononuclear fraction. Two different normal marrow donors gave identical results.
D. MAP3K8 expression levels are elevated in myeloma cells compared to non-lymphoid and non-haematopoietic tumour cells. MAP3K8 transcript expression data in multiple myeloma, leukaemia, lymphoma and solid tumour cell lines. Results are depicted in boxplots, which highlight the median and interquartile range of expression of MAP3K8 in each group. The whiskers of each boxplot represent the minimum and maximum expression of MAP3K8 for the respective group. The values for the log2-transformed median-centered expression of MAP3K8 transcript among myeloma cell lines was higher compared to solid tumours (p<0.0001, Kruskal-Wallis test for one-way analysis of variance, and p<0.05, post-hoc Dunn's multiple comparison test).
E. MAP3K8 protein expression and ERK activation in primary myeloma tumour cells. Lysates from primary CD138+ myeloma tumour cells were immunoblotted using antisera against total MAP3K8, phospho-ERK, total ERK and GAPDH as a loading control. In myeloma cells, the p58 MAP3K8 isoform was preferentially expressed.
F. MAP3K8 promotes ERK and JNK activation in response to TNF receptor stimulation in myeloma cells. MM1.S cells were serum-starved overnight in the presence or absence of MAP3K8 inhibitor (5 µM). A 5-min pulse of recombinant human TNFα (50 ng/ml) was given at the end of the incubation and cells were immediately lysed in protein buffer. MAP3K8 inhibition resulted in a reduction of phospho-ERK and phospho-JNK induction following stimulation with TNFα whereas p38MAPK activation status remained unaffected.
G. Treatment of myeloma cells with a small-molecule MAP3K8 kinase inhibitor results in dose-dependent reduction in phosphorylation of the MAP3K8 direct substrate, MEK. U266 myeloma cells were incubated with escalating concentrations of MAP3K8 inhibitor for 24 h in the presence of continuous mitogenic stimulation (10% fetal calf serum). Whole cell lysates were subsequently immunoblotted and probed with antisera against phospho-MEK1/2 and total MEK1/2. DMSO, dimethyl sulfoxide.