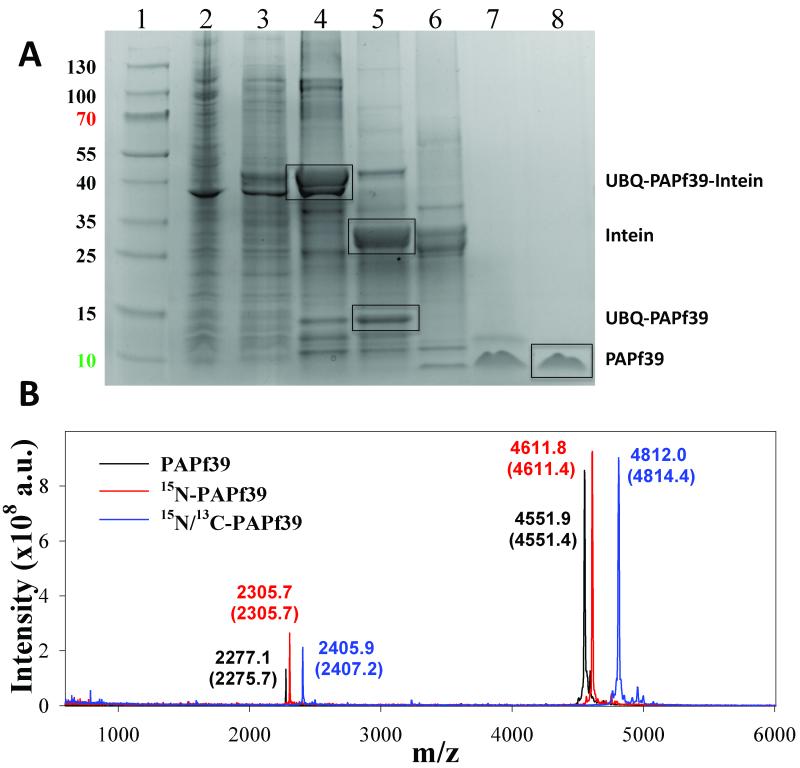
Figure 2.
Purification of the recombinant PAPf39 peptide. Panel A. Coomassie blue stained SDS-PAGE analysis of recombinant soluble lysates in E. coli strain BL21(DE3): (Lane1: Thermo Scientific Page Rules Prestained Protein Ladder 10-130 kDa) (lane 2: whole cell lysate), (lane 3: soluble protein fraction), (lane 4: fractions after Ni-NTA), (lane 5: cleavage of the intein), (lane 6: TEV-protease digest), (lane 7: PAPf39 containing fraction after SEC), (lane 8: pure PAPf39 fraction after RP-HPLC). The position of migration of all fragments, including PAPf39, is shown. Panel B. Mass spectrometry analysis of purified PAPf39: unlabeled PAPf39 (black line), 15N-labeled PAPf39 (red line), and 15N/13C-labeled PAPf39 (blue line). Determined masses are shown above each peak, with expected masses given in parenthesis.