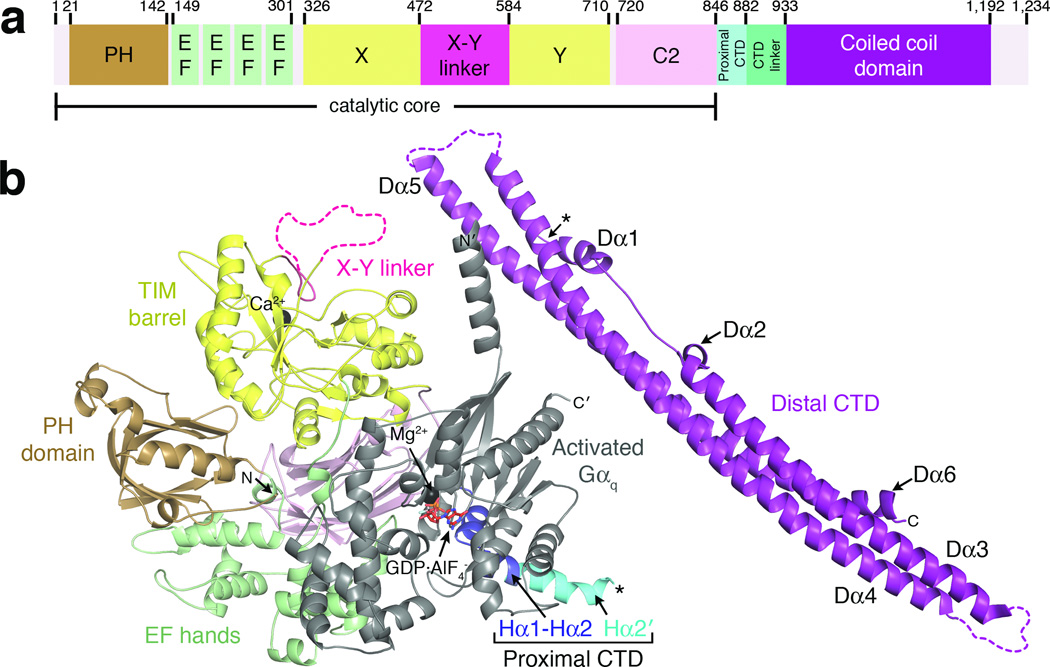
Figure 1.
Crystal structure of Gαq–PLCβ3 reveals the C-terminal coiled-coil domain (distal CTD) in the context of a fully active signaling complex. (a) Primary structure of human PLCβ3. Numbers above the diagram correspond to domain boundaries. The catalytic core of the enzyme is defined as extending from the N-terminus to the end of the C2 domain. (b) A monomer of Gαq–PLCβ3 from the asymmetric unit. Domains of PLCβ3 are colored as in (a). The observed C-terminus of the proximal CTD and the N-terminus of the distal CTD are marked with black asterisks, although this represents only one possible linkage between the catalytic core and distal CTD in the crystal lattice (see Supplementary Fig. 4). The observed N and C-termini of PLCβ3 and Gαq are labeled N and C, and N' and C', respectively. Ca2+ and Mg2+ are shown as black spheres. Activated Gαq is shown in gray, with bound GDP and AlF4− drawn as red sticks. Dashed lines correspond to disordered loops.