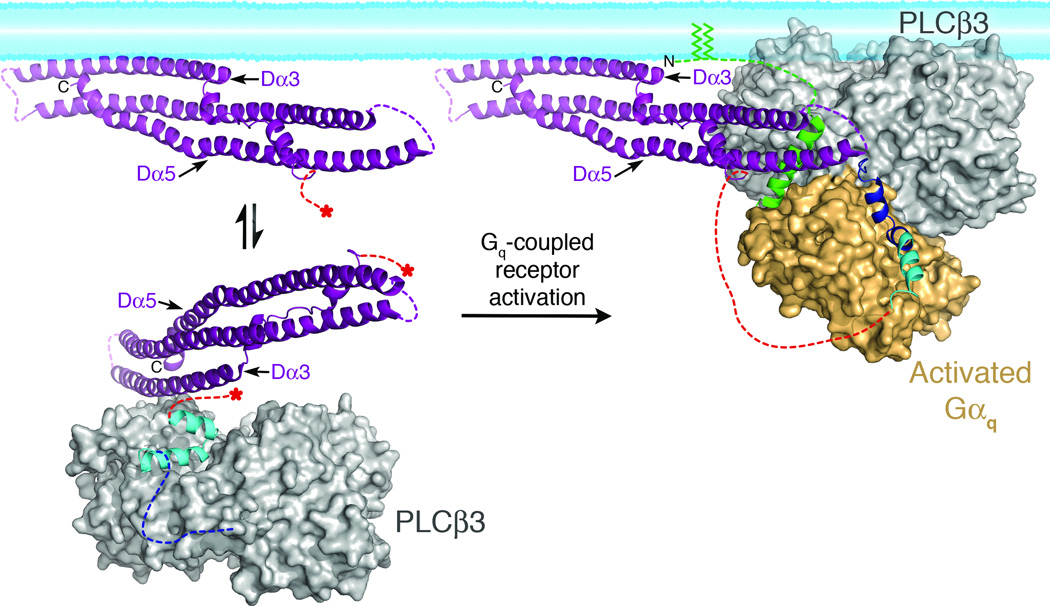
Figure 5.
Model of PLCβ3 regulation by the distal CTD and Gαq. In the absence of Gαq (left), the Hα2' inhibitory helix from the proximal CTD (cyan), is bound to the catalytic core (gray surface), stabilizing the enzyme in a more quiescent conformation (based on PDB entry 3QR0)19. The primary Gαq binding site (dashed blue line) is disordered in this state. The distal (purple) and proximal CTDs are connected by the disordered CTD linker (dashed red lines). Red asterisks mark the start and end of the linker in the basal state. The distal CTD could help partition the enzyme between membrane-associated and cytosolic populations by forming a transient interaction with the hydrophobic ridge of the catalytic core (see Fig. 4b, d). Upon activation, Gαq binds to the Hα1-Hα2 helical hairpin (blue), displacing Hα2' from the catalytic core. The hydrophobic patch of the distal CTD engages the N-terminal helix of Gαq (green), and helps orient the catalytic core of PLCβ with respect to the lipid bilayer. The palmitoylated cysteines of the Gαq N-terminus (green lines) also help anchor the complex at the membrane. This model was created by independently docking the N-terminal helix of Gαq in complex with the distal CTD, and the remainder of Gαq in complex with the PLCβ3 core at a common membrane surface, then translating the distal CTD complex such that the Gαq N-terminal helix makes a reasonable connection to the rest of Gαq. The model also brings the PH domain in close proximity to the membrane, consistent with studies indicating this domain also contributes to membrane binding44,47.