Abstract
Evidence from varied community settings has shown that the Group Lifestyle Balance (GLB) Program and other adaptations of the Diabetes Prevention Program (DPP) intervention are effective in lowering diabetes risk. Most DPP data originated from studies of pre-diabetic whites, with only sparse evidence of the effect of DPP in African Americans (AAs) in community settings. This paper describes the design, methods, baseline characteristics and cost effective measures, of a single-blinded, cluster- randomized trial of a faith-based adaptation of the GLB program, Fit Body and Soul (FBAS). The major aims are to test efficacy and cost utility of FBAS in twenty AA churches. Randomization occurred at the church level and 604 AA overweight/obese (BMI≥25 kg/m2) adults with fasting plasma glucose range from normal to pre-diabetic received either FBAS or a health-education comparison program. FBAS is a group-based, multi-level intervention delivered by trained church health advisors (health professionals from within the church), with the goal of ≥7% weight loss, achieved through increasing physical activity, healthy eating and behavior modification. The primary outcome is weight change at 12-weeks post intervention. Secondary outcomes include hemoglobin A1C, fasting plasma glucose, waist circumference, blood pressure, physical activity level, quality of life measures, and cost-effectiveness. FBAS is the largest known cohort of AAs enrolled in a faith-based DPP translation. Reliance on health professionals from within the church for program implementation and the cost analysis are unique aspects of this trial. The design provides a model for faith-based DPPs and holds promise for program sustainability and widespread dissemination.
Keywords: African-American, church, cluster, translation research, diabetes prevention, obesity
1. Introduction
Type 2 diabetes (T2DM) accounts for 90 to 95 percent of United States (US) diabetes cases [1]. Risk factors include older age, family history of diabetes, previous history of gestational diabetes, and physical inactivity, however weight is the risk factor most associated with incident diabetes [2]. The US Diabetes Prevention Program (DPP) established that lifestyle modification targeting weight loss is efficacious in reducing incident T2DM by 58% over 3 years [3]. Multiple DPP lifestyle intervention adaptations, including the Group Lifestyle Balance (GLB) program, were effective in lowering diabetes risk factors [4, 5, 6, 7, 8, 9]; however, evidence of implementation of such programs in African American (AA) populations is sparse.
African Americans compared to non-Hispanic whites, experience higher rates of T2DM (12.6%: 7.1%) [10]. Disparities in rates of diabetes are partly attributed to the disproportionately higher prevalence of overweight and obesity among AAs compared to whites (76.7%; 66.7%) [11]. While US obesity trends are leveling off in other races, obesity continues to trend upward among AAs [11]. Despite disparities, much of the current DPP translation evidence originates from samples primarily of pre-diabetic non-Hispanic whites [12]. Given the high level of T2DM risk among AAs, the generation of a more robust body of evidence regarding successful translation of DPP interventions in this population may have the potential to improve the public’s health.
The church is an effective setting to gain access to AAs for health promotion research [13, 14]. Churches play a major role in AA communities, often providing support for spiritual, financial and social needs. Many AA churches consider health as part of their mission and have well-organized multidisciplinary health ministries [15]. A church health ministry is a group of health professionals and lay volunteers that integrates faith and health within the congregation and provides health-promoting activities such as health screenings and health education [16]. Due to their commitment to health promotion within their individual churches, health ministries could play an integral role with DPP translation research conducted within AA churches.
Published AA church-based DPP trials to date have primarily been small non-randomized feasibility trials, ranging from 10 – 40 participants [17, 18, 19]. Moreover, the comparative effectiveness AA church-based DPP trials had limited success in enrolling large samples (n=37–246) [20, 21]. Although researchers have used lay community health workers to recruit participants and deliver a DPP translation intervention through AA churches [21], to our knowledge there are no DPP randomized efficacy trials in which researchers have trained the professional health ministry members to deliver the entire intervention. In addition, investigators have not generated sufficient evidence regarding the cost utility of DPP community-based translation [4, 5, 6, 7, 8].
The purpose of this paper is to describe the design, methods, baseline characteristics and cost effective measures of a church-based cluster randomized trial, Fit Body and Soul (FBAS). The aim of FBAS is to test the efficacy and cost utility of the adapted GLB program implemented by church health advisors in Southeastern US AA churches, compared to a health education program.
2. 0 Study Development
The recommendations for the AA church collaborating with Georgia Health Sciences University [GHSU] (formerly the Medical College of Georgia) to implement a diabetes prevention program originated from GHSU’s Health Disparities Community Advisory Board (CAB). Members of the CAB, which includes local pastors, nurses, and other community leaders, identified obesity and diabetes as priority health conditions for the local AA community. The pastors on the CAB expressed a desire to have a community-led diabetes prevention program offered through the local churches.
The investigators along with representative community partners sought to develop the project within the framework of community based participatory research [22]. Our multi-disciplinary academic research team includes a nurse-scientist who is a trusted AA faith- leader. Based on the CAB recommendations, the nurse scientist and other research team members met with other local AA pastors and health ministry members to determine interest, garner additional support and input on a collaborative project. Due to the structured group-based delivery format of the GLB program and the growing evidence of success of DPP translation, the investigators chose to use the GLB curriculum as the basis for building the FBAS intervention [6, 23]. With high local interest, we formed a project-specific advisory group comprised of the research team, DPP experts from the University of Pittsburgh Diabetes Prevention Support Center [24]; an expert from another church-based trial (Body and Soul) [25]; and a pastor and health ministry members from six local churches, which were identified by CAB members as churches having active health ministries. The advisory group provided input for all aspects of the planned project, including recommendations to modify the GLB curriculum by adding selected scriptures, socio-cultural preferences, AA graphics, and quotes from famous AAs [17]. The advisory group also suggested the project name as Fit Body and Soul.
With preferences from both the community and investigators to address the multiple layers of influence within the church, we adopted the Socio-ecological Model as the theoretical framework for project implementation [26]. The advisory group provided input on the best methods for integrating the multiple levels (i.e., individual, group, organizational, congregational, policy) into the context of the church, as well as recommendations regarding project protocols. Based on these formative assessments, investigators developed and implemented a 12-week feasibility pilot study in one church [17, 27]. Of the 40 participants enrolled, 35 (87%) attended at least 10 sessions and completed all data collection. Of these 35 participants, 48% lost at least 5% of baseline weight, 26% lost 7% or more, and 14% lost >10% of baseline weight [27]. Results indicated that successful translation of the DPP to a faith-based setting was feasible and potentially effective. We obtained additional funding to test the faith-based translational model in a larger randomized control trial, which is the focus of this paper.
3.0 Methods
3.1 Design of the Fit Body and Soul Study
This study is a single-blinded, cluster-randomized, community-based trial. Investigators allocated churches to two arms: the FBAS (intervention arm) which is a faith-based adaptation of the GLB intervention, and a health education program (comparison arm) developed from the list of topics provided by the Centers for Disease Control and Prevention (CDC) Guide to Community Prevention Services [28, 29]. We recruited 20 churches located in a Georgia metropolitan area, which includes both urban and rural communities. We assumed that churches of similar size would share similar resources and church culture; we therefore stratified the churches by membership size and then randomized them in pairs at the church level to receive the FBAS intervention or the comparison intervention. The study began with participant recruitment and training of church health advisors (CHAs) at the first church in September 2009, followed by baseline data collection in October 2009. Investigators completed baseline assessment for all twenty churches in March 2012. Final data collection will occur by April 2013. Georgia Health Sciences University’s Institutional Review Board, known as the Human Assurance Committee, approved the study.
3.2 Church Recruitment
Investigators recruited churches from August 2009 to November 2011. The criteria for church inclusion were: 1) pastor or church administrator report of AA-predominant membership of 200 or more, 2) an existing health ministry and/or at least four health professionals within the church who were willing to lead the sessions, and 3) a pastor willing to accept church randomization. Due to the large number of churches in Richmond County, Georgia, we targeted churches that were: 1) known by the nurse scientist or another research team member or, 2) recommended by a member of the CAB. Additionally, a CAB pastor who was the leader of the local ministers group allowed research team members to attend the ministers’ meeting to present the project and recruit churches. The nurse-scientist or study coordinator then contacted each church to arrange an appointment with the pastor. With a recruitment goal of 20 churches, we met with the pastors from 28 of 35 churches who had expressed interest. After the pastors agreed to participate, the investigators requested a letter of support. The letter included the following: the church’s intent to participate in the study, the church membership size, and a brief description of the pastor’s understanding of the study. Church recruitment continued until investigators had obtained signed letters of support from 20 pastors. We placed the names of the churches that we did not meet with (n=7) on a list of potential churches.
Of the pastors who did not agree to participate (n=8), reasons included the lack of health professionals available to lead the sessions (n=3), a busy church calendar, and one pastor’s belief that health promotion activities did not align with the current mission of the church. The pastors of two churches initially agreed, but did not respond to follow-up phone calls; and the pastor of one church stated that the church lacked sufficient members who were willing to participate. All of the included churches were of the Christian faith. Church congregation size ranged from 200 to 3000; 12 (60%) had less than 1000 members; four (20%) between 1000 and 2000; and four (20%) had 2000 – 3000 members. Each church received $1,500 compensation for participating in the study and for providing the space for group sessions and data collections. Churches entered the study in six cohorts, each cohort including either two or four churches. A new cohort began every four months. To maximize early participant enrollment, we chose to begin with the larger churches. Investigators informed the pastors of the overall project timeline and the anticipated start date at their church. To keep the pastors engaged and abreast of the study timeline, we invited them to CAB meetings and periodically sent them study update letters.
Prior to the start of a new cohort, investigators met again with each pastor of the upcoming cohort. During the meeting, the investigators informed the pastor of the results of randomization, confirmed the start date of participant recruitment, intervention start dates, session days and times, and data collection dates. Additionally, the pastor signed a memorandum of understanding, which described the responsibilities of the church, the CHAs and the research team. The memorandum also described the incentives for the participants, CHAs and church.
3.3 Participant Inclusion and Exclusion Criteria
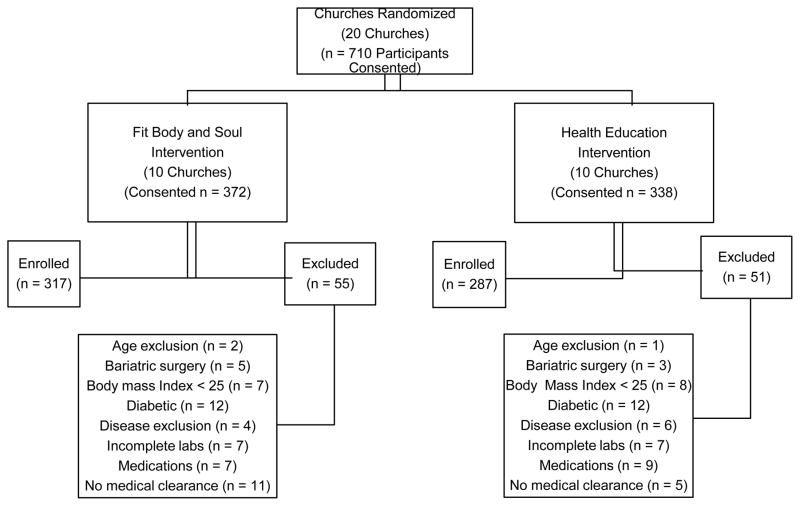
Table 1 depicts participant inclusion and exclusion criteria. Inclusion criteria were selected to include AAs who were non-diabetic but at risk for developing T2DM. Exclusion criteria were medical contraindications to physical activity, physical conditions or medications that might affect glucose metabolism, behaviors that may interfere with participation and illnesses that would limit life span. Investigators consented 710 participants and excluded 106, which resulted in a final enrollment of 604 participants. Figure 1 shows the number of consented participants by intervention arm and assessed for eligibility, the reasons for exclusion, and the final number enrolled from the 20 churches.
Table 1.
Inclusion and Exclusion Criteria for Participant Enrollment.
| Inclusion Criteria | |
|---|---|
| Selected Characteristics | Age 20-64 years of age |
| Self-described African American | |
| BMI ≥25kg/m2 | |
| Planning to remain within the community for 1 year | |
| Non-diabetic | |
|
| |
| Exclusion Criteria | |
|
| |
| Glucose Status | Fasting plasma glucose ≥ 126 mg/dl following at least an 8-hr fast or A1C≥7.0% |
|
| |
| Certain physical conditions | Human immunodeficiency virus/Acquired immune deficiency syndrome |
| Active tuberculosis | |
| Cancer requiring treatment in past 5 years except for cancers that have been cured or in the opinion of the researchers has a good prognosis | |
| Stroke within the past 6 months | |
| Cirrhosis of the liver | |
| Currently pregnant or planning pregnancy within the study period | |
| Gastric weight-loss surgery | |
| Weight loss > 10% in past three months for any reason other than childbirth | |
|
| |
| Conditions that may confound treatment effects | |
|
| |
| Medications | Anti-diabetic medications |
| Prescription weight- loss medications | |
| Anti-neoplastic agents | |
| Anti-psychotic agents that have glucocorticoid effect | |
| Oral corticosterioid use >6 weeks | |
|
| |
| Conditions or behaviors likely to affect the conduct of the intervention | Unwilling or unable to give informed consent |
| Unable to communicate with the church team or research group due to cognitive matters | |
| Participation in another research study that would interfere with Fit Body and Soul | |
Figure 1.
Participants Allocated, Consented, Screened, and Enrolled.
3.4 Participant Recruitment
Recruitment began at each church 4–6 weeks prior to intervention start. The pastors agreed to endorse the project during Sunday service and used a standardized script encouraging congregational participation. Study- trained CHAs distributed investigator-developed flyers to church members and made scripted-podium announcements to promote the study. Members of the research team often attended worship services and bible study to assist with recruitment as well as to provide information regarding the study.
3.5 Participant Compensation and Efforts to Retain Participants
Individual participants in both study arms received: 1) a t-shirt at baseline data collection; 2) a participant binder and study handouts; 3) a $25 gift card for attending week 6 group session; 4) a pedometer at Session 10; 5) a $50 gift card from a national retail chain for completing the 12- week data collection; 6) a $75 gift card at the 12-month data collection; and, 7) chances to receive low-cost door prizes, such as picture frames, CDs, and DVDs from drawings conducted at the sessions.
Prior to beginning the study, we consulted with the respective pastors, CHAs, and church administration to determine a mutually acceptable time to conduct each session and data collection. To enhance participant retention and complete data, the CHAs contact each participant to remind them of the upcoming session. CHAs also contact participants that were absent from sessions to assess barriers to attendance. Each week during the intervention period, the church announcements highlight the topic of the upcoming session. Participants report to their respective churches where trained data collectors and contracted phlebotomists obtain all data on the designated Saturday mornings. Phone calls and letters from the research assistants remind each participant of scheduled data collections. Participants unavailable to attend church data collections have their data collected at Georgia Health Sciences University and phlebotomy at a local laboratory.
3.6 CHA Training and Compensation
As master trainer for the FBAS intervention, a co-investigator completed motivational-interviewing (MI) training and a two-day GLB training workshop provided by the Diabetes Prevention Support Center [24]. The master trainer then trained four health professional members (e.g., nurses, pharmacists, physicians) from each FBAS intervention church.
The FBAS intervention and comparison intervention CHAs began their respective training [by church cohort] within 4 weeks prior to delivery of session one. The FBAS intervention training used didactics, observation, experiential learning, and peer feedback. CHAs involved in the FBAS intervention received two separate 8-hour training sessions and were compensated $200 for the training. Training day one provided the following: an overview of the results from the original US DPP, training on FBAS recruitment methods and study protocols; discussion regarding study logistics such as classroom space and potential scheduling conflicts with other church activities. Additional day one training included MI techniques, integration of faith concepts, and group facilitation techniques such as building group cohesion. Training also covered weigh-in procedures, guidelines for review of participant food diaries, and participant telephone contacts. Finally, training covered specific delivery of core sessions 1–6 of the FBAS intervention.
The master trainer attended each of the first four FBAS sessions and provided feedback and further coaching. Training day two occurred after the CHAs had delivered the first four intervention sessions. During day two training, the trainer reinforced earlier feedback, addressed CHA concerns regarding any aspect of the study such as study protocols and participant concerns. Additionally, the trainer reviewed MI techniques and provided specific training on the delivery of core sessions 7–12 as well as the six post-core sessions.
Comparison intervention CHAs received 8 hours of training taught by two research team members with public health backgrounds. The training of the CHAs for the comparison intervention differed in the intervention content and there was no MI content. Comparison intervention CHAs were compensated $100 for the training session.
CHAs from both arms used a scripted manual to deliver all group sessions. As deemed necessary by the trainers, both groups of CHAs periodically received ongoing training reinforcement and content review throughout the intervention period. At session 12, all CHAs are compensated $500 and at the sixth post-core session; all CHAs are compensated $250. Seventy-one percent of the trained CHAs were nurses; the remaining CHAs were pharmacists, respiratory therapists, health professional technologists and a medical doctor.
4.0. Interventions
4.1 FBAS Intervention
The FBAS intervention is a modified version of the University of Pittsburgh-developed Group Lifestyle Balance (GLB) curriculum. Investigators at the University of Pittsburgh developed the original DPP, a clinic-based, individually- administered intervention [30]. Social Cognitive Theory informed the DPP, which was an intensive structured 16-session core-curriculum. During the DPP, clinicians delivered behavioral strategies such as dietary and physical activity self-monitoring, participant self-weighing, goal-setting and behavioral modification for weight loss and physical activity [31]. Investigators subsequently updated and modified the DPP to a group format, which is the GLB program [28]. The FBAS intervention maintained fidelity to the GLB curriculum; moreover, in addition to the adaptations made with the study advisory group, we tailored the curriculum further following completion of the pilot study. In consultation with a motivational-interviewing (MI) expert, we integrated MI themes, such as open-ended questions and CHA assessments of participants’, values, confidence and motivation for lifestyle change [32].
FBAS is a 12-week behavioral intervention with two defined goals: 1) weight reduction of ≥7% of initial weight, and 2) physical activity of ≥ 150 minutes per week of brisk walking. Participants attend 12-weekly group one-hour core sessions at their respective church. CHAs deliver the weekly sessions followed by six monthly one-hour post-core sessions. The 12 core sessions comprise the core components of successful weight loss programs such as strategies to reduce calories and dietary fat consumption, encouraging physical activity, and behavioral modification such as stimulus control, goal setting, and problem solving.
Participants receive a binder at session one and research team members deliver handouts at each session. Beginning at session one and throughout the intervention, CHAs emphasize the importance of dietary self-monitoring and provide participants with a self-monitoring log. Participants return their completed logs during the weigh-in that occurs prior to each session. During the weigh-in, CHAs use a scripted-MI phrase to motivate participants to continue lifestyle changes and to identify participant barriers. During the weigh-in, the CHAs return the participant’s previous food and activity log in which they have reviewed and made encouraging notations.
Weekly during the 12 core sessions and monthly thereafter the CHAs telephone each participant to make individual contact. During the brief call, the CHAs review the participant’s food and activity log and use scripted MI messages to address participant barriers to lifestyle changes.
CHAs introduce physical activity at week four and thereafter participants are encouraged to self-monitor and record physical activity lasting 10 minutes or longer. CHAs provide participants with a pedometer at week 10 after which the participants record daily steps. Table 2 outlines the FBAS multi-level intervention components.
Table 2.
Multi-level Activities of FBAS Intervention
| Week | Core Sessions | Individual Level (led by Church Health Advisor) | Group Sessions (led by Church Health Advisors) |
|---|---|---|---|
| 1 | Welcome to FBAS: Getting Started Losing Weight |
Build individual commitment. Assessment of personal values, motivation and confidence levels. Identify personal weight loss goals. |
Build group commitment; introduce intervention goals (7% weight loss and 150 minutes/weekly PA activity; introduce self monitoring of food intake. |
| 2 | Be a Fat & Calorie Detective | Introduce self-monitoring of wt. (i.e., assign fat gram goal based on initial weight). Reinforce self-monitoring as key behavioral modification. | Introduce self-monitoring of fat and calorie intake; instruct on reading food labels; provide fat and calorie counting book. Introduce behavioral modifications to reduce fat and calorie intake. |
| 3 | Healthy Eating | Practice self-monitoring skills (i.e., measuring foods, estimating food portion size). Reinforce diet behavioral modification. | Teach components of a healthy meal. Teach healthy meal planning. |
| 4 | Move Those Muscles | Add PA to self-report. Assess personal PA likes and dislikes. | Introduce physical activity. Advise of plans to gradually build to 150 minutes per week. Begin PA self-monitoring. |
| 5 | Tip the Calorie Balance | Provide an individually tailored meal plan at reduced calorie levels. Reinforce self-monitoring |
Teach the fundamentals of energy balance and necessary actions to lose 1–2 lbs. per week. Self-evaluation of progress. |
| 6 | Taking Charge of What’s Around You | Identify cues in individual’s environment and develop action plan. | Introduce the principle of stimulus control; identify cues in the environment that lead to unhealthy food and activity choices, discuss behavioral strategies to overcome cues. |
| 7 | Problem Solving | Apply the problem- solving model to eating and physical activity problems; develop an action plan. | Present the 5-step model of problem solving. Make an action plan and evaluate success solutions. |
| 8 | Four Keys to Healthy Eating Out | Develop eating out action plan. Reinforce self-monitoring. |
Introduce four basic skills for managing eating away from home. |
| 9 | The Slippery Slope of Lifestyle Changes | Discuss personal triggers; develop action plan for managing slips. | Stress that slips are normal and learning to recover quickly is the key to success. Teach participants to recognize personal triggers for slips, their reactions to slips, and behavioral strategies to resume lifestyle change after a slip. Self-evaluation. |
| 10 | Jump Start Your Activity Plan | Reinforce positive behaviors. | Introduce the pedometer and instruct on use. Issue pedometers. Review ways to boost activity. Teach to identify time to include short bouts of PA. |
| 11 | Make Social Cues Work for You | Develop action plan for social cues. Trouble-shoot problems with pedometer. |
Teach how to identify social cues, behavioral strategies to change social cues, strategies to add positive social cues. |
| 12 | Ways to stay motivated | Develop action plan for self- motivation. | Review progress. Teach ways to stay motivated and manage stress. Setting new life-style goals. |
| Month | Post-Core Sessions | ||
| 1 | Bulk Up Your Diet | Assess barriers encountered during last 4 wks. Encourage periodic continued self-monitoring. | Continue self-monitoring. Teach strategies for adding volume to meals. |
| 2 | Regulate Your Mind | Assess efforts to increase dietary bulk. | Review reasons for seeking weight loss. Practice countering excuses. Overcoming barriers. Review strategies to manage diet and PA slips. |
| 3 | Muscle Matters | Assess use of strategies to manage lifestyle setbacks. | Introduce safe use of resistance bands, flexibility, and balance activity. Issue resistance band. Develop a resistance activity plan. |
| 4 | Too Blessed to be Stressed | Review safe use of resistance band. | Review the barrier of stress; teach ways to manage stress including sleep and laughter. |
| 5 | The Inside Scoop on Indoor Activity | Assess use of stress management techniques and addition of resistance exercises. | Teach ways to stay active indoors. Exercise session with DVD. |
| 6 | Pressing Forward Toward Long-term Weight Maintenance |
Encourage continuation of lifestyle modifications. | Review shifts in thinking patterns of those successful at weight reduction. |
Abbreviations: FBAS-Fit Body and Soul; PA-physical activity; MI-motivational interviewing; wks-weeks;
Notes: Tailored individual-level intervention using MI to assess barriers and reinforce behavioral modification occurs during weigh-ins, weekly and monthly phone calls. Self-monitoring logs collected and previous log returned during private weigh-ins. Organizational and congregation- wide events implemented throughout the intervention include: pastor announcements of total weight loss, announcements of upcoming session, pastor recognition of participants, congregation-wide health event such as speaker, grocery shopping trip, cooking demonstration or physical activity class. Policy change implementations such as: change in types of meals served, change in vending machine items, etc.
CHAs schedule post-core session one to occur 4 weeks after the core sessions end. The six monthly FBAS post-core sessions are included in order to maintain participant engagement, and to promote continued progress toward weight goal and/or weight maintenance. The post-core sessions expand on the earlier weekly sessions and include topics related to dietary intake, physical activity, resistance training, and behavior modification.
Each FBAS session includes a private weigh-in, (on a digital bathroom scale provided by research team) followed by a group session. Each group session begins with prayer, followed by discussion of relevant scriptures and, presentation of a new topic, ongoing identification of barriers to weight loss and activity; and development of group action plan/goals for the next sessions. Participants receive intervention-enhancing materials such as weekly food and activity diaries, a fat and calorie counter book at session two, a pedometer, and a resistance band and accompanying resistance-training booklet at post-core session three. Each session ends with the participants standing and reciting a motivational scripture.
CHAs tailor the other multi-level activities (organizational, congregational and policy change) to their respective church. Organizational level activities are those that CHAs and pastors integrate within the usual activities of the church. These include activities such as podium announcements that emphasize the FBAS program, health messages during Sunday service, prayers for FBAS participants and announcements of the total group weight loss. Congregational-level activities engage the entire congregation in health-related activities. Examples include cooking demonstrations and invited health speakers. In addition to the multi-level activities, each FBAS intervention church agrees to make one health-related policy change. Examples of policy change activities include the church setting guidelines about the types of food served at church functions and adding healthy choices in the church vending machines. The CHAs document all session weigh-in data and the multi-level church activities. At the end of the intervention period at each church, the research assistants collect the CHA documentation; these data, however, are not included in the analysis.
4.2 Comparison Intervention
Based on input from the CAB regarding acceptability to our target population, we included a comparison intervention rather than a control. The comparison intervention addresses key health issues facing AAs in Richmond County, Georgia (Table 3), investigators selected the content from a list of health topics provided by the Centers for Disease Control and Prevention (CDC) Guide to Community Prevention Services [29]. We developed the topics into a scripted manual and developed participant handouts from information provided by the American Heart Association, American Cancer Association, American Diabetes Association, Mental Health America, and other national organizations. During the 12 weekly sessions and the six monthly sessions, the CHAs begin with prayer, and then lead group discussion regarding the health topics; CHAs also give the participants assignments to assess their risk for the discussed condition. We included the six monthly sessions to maintain participant engagement and to keep the intervention dose and attention consistent among the two study arms. During the six monthly sessions, the CHAs review the original 12-session material (now merged into six sessions), and facilitate the course of discussions.
Table 3.
Content of Health Education Comparison Group Sessions.
| Week | Major Theme | Group Session (led by Church Health Advisors) |
|---|---|---|
| 1 | Introduction | Overview of program. Reasons to maintain health and assessment of health risks |
| 2 | Mental Health and Stress | Stress management strategies and indications to seek mental health care |
| 3 | Heart Disease and Stroke | Modifiable and non-modifiable risk factors. Signs of a heart attack and stroke |
| 4 | Diabetes | Identify types of diabetes; risk factors, signs and symptoms and preventive strategies |
| 5 | Cancer | Common types; risk factors; signs and symptoms; ways to detect and prevention of cancer |
| 6 | Smoking | Health impact of smoking on smoker and second hand smoke, social action, and cessation techniques |
| 7 | Injury and Violence | Role of injury on health, common injuries and preventive strategies. |
| 8 | Asthma | Identify risk factors, signs and symptoms, diagnostic criteria, treatment, and emergency asthma symptoms |
| 9 | Nutrition | Identify caloric needs, types of nutrients in a healthy diet, reading food labels, and healthy eating |
| 10 | Physical Activity | Identify benefits of PA, recommended weekly PA-levels, types of PA, strategies to meet activity goals, injury prevention. Instruct on pedometers and distribute pedometers |
| 11 | HIV/AIDS | Differences between HIV and AIDS, HIV transmission, risk factors, prevention, signs, symptoms and treatment |
| 12 | Communicating with Your Healthcare Provider | Effective communication with health team and low cost community health care |
| Month | Post-Core Sessions | |
| 1 | Review health risks and Mental Health | Review key content of combined sessions using questions and sharing. |
| 2 | Review Heart Disease & Stroke and Diabetes | Review key content of combined sessions using questions and sharing. |
| 3 | Review Cancer and Smoking | Review key content of combined sessions using questions and sharing |
| 4 | Review Injury & Violence and Asthma | Review key content of combined sessions using questions and sharing |
| 5 | Review Nutrition and Physical Activity | Review key content of combined sessions using questions and sharing |
| 6 | Review HIV/AIDS & Health Communication | Review key content of combined sessions using questions and sharing |
4.3 Intervention Fidelity
A research team member attends each group session to record participant attendance and to conduct fidelity monitoring using an investigator-developed fidelity tool to verify that the content delivered by CHAs is as designed with all core components delivered in the appropriate manner and context to the appropriate group. The fidelity tool lists the essential components of the specific session. In the event that the CHA omits a key component, the research team member redirects the CHA to the content.
5.0 Data Collection
5.1 Assessments
At the baseline visit, investigators obtain informed consent and determine participant eligibility. Potential participants bring current medications to baseline and 12-month data collection. A physician investigator reviews the medication list to assess participant eligibility (See Table 1 for a list of exclusion medication classifications). Clinician investigators use the Physical Activity Readiness Questionnaire (PAR-Q) [33] to determine if a potentially eligible participant from both study arms needs a physician’s approval to participate. Based on affirmative answers to specific health questions such as chest pain or shortness of breath, the clinician investigators provide the participant with a physician approval letter and instruct participants to return the completed letter prior to the physical activity portion of the intervention (week four). Participants are withdrawn from the study if they do not provide the approval letter. Figure 1 details the success of this process.
Prior to the initial data collection and when new data collectors join the team, a study investigator uses a common standardized operating protocol to train all data collectors who are investigators, research staff, students, and campus volunteers (blinded to the intervention). Training includes a detailed review of all data collection protocols. Trainees observe a simulated data collection and then perform return demonstrations of each measure. During data collections, the data collector trainer periodically reassesses each data collector for adherence to protocols and measurement drift.
At baseline, data collectors obtain demographic data, anthropometric, physiological measures and self-administered questionnaires. Phlebotomists contracted through a local laboratory collect blood for biochemical measures. Data collectors obtain all data (except height) again at 12–14 weeks post-baseline and 12 months post-baseline. Although the intervention period is 9 months, to be consistent with the original DPP and other DPP translation studies, we schedule the final data collection to occur 12 months post-baseline [4, 5, 30]
5.2 Anthropometric Measures
Weight (in kilograms), and height (in centimeters) are measured with the participant standing erect and looking straight ahead while in light-weight clothing and without shoes using the calibrated Seca 703 high-capacity digital scale fitted with the Seca 220 height rod. Data collectors measure waist circumference (WC) with the participant standing, with feet flat on the floor at shoulders width apart. The abdomen is fully exposed and using the Gulick II non-stretching tape with a tensioning mechanism the data collector locates the mid-point between the lowest palpable rib and the iliac crest regardless of umbilical location, measures the WC and records the measurement to the nearest tenth of a centimeter.
5.3 Cardiovascular Function
Data collectors use an appropriately sized cuff on the participants’ right upper arm to collect blood pressure and resting heart rate with a calibrated blood pressure monitor (Dinamap Procare 420). After the participant sits quietly for five-minutes with back and right arm supported and feet flat on the floor, the data collector assesses and records two blood pressure measurements taken one minute apart. The averaged readings are analyzed as the blood pressure.
5.4 Biochemical Measures
Phlebotomists from a contracted local laboratory attend all data collections at the respective churches and collect venous blood for laboratory measures (FPG, A1C and hemoglobin) from participants after overnight fasting. The laboratory determines FPG concentrations by a modified hexokinase enzymatic method, and A1C by a high-performance chromatography certified by the National Glycohemoglobin Standardization Program and standardized to the Diabetes Control and Complications Trial (personal communication, P. Williams, Clinical Pathology Laboratories Southeast, Augusta, GA, 2010). In accordance with the American Diabetes (ADA) guidelines, we define a FPG of greater than 126 mg/dl or an A1C of 7% or more as diabetes [34]. Although hemoglobin is not an outcome measure, we obtain it to assess for known confounders of A1C results such as anemia and hemoglobin variants [35].
5.5 Physical Activity
We use the self-administered International Physical Activity Questionnaire long form (IPAQ-LF) to measure physical activity [36]. The IPAQ-LF comprehensively assesses moderate-intensity and vigorous-intensity activities performed across four domains: leisure time, work, domestic and gardening (yard) activities, and transport. The IPAQ-LF measures sedentary behavior by a single item that assesses time spent sitting on weekdays and weekends. The IPAQ-LF allows calculation of metabolic equivalent of task (MET) which is the energy expenditure of a specific activity. METs can be analyzed as either a continuous variable of MET-minutes/week or as a categorical variable (low, moderate, high physical activity levels) based on IPAQ-LF specified scoring.
6.0 Quality of Life Measures
The 12-item survey, SF-12 version 2 (SF-12v2®) is the self-administered validated tool used for measuring health-related quality of life [37]. The tool covers eight domains: (1) physical functioning, (2) role physical, (3) bodily pain, (4) general health, (5) vitality, (6) social functioning, (7) role emotional and, (8) mental health. We will use the Norm-Based Scoring noted in the SF-12v2® to compare the eight–scale scores and the two summary measure scores (Physical Component Summary and the Mental Component Summary). Norm-Based Scoring consists of standardizing each SF-12v2® scale using a z-score transformation. A linear z-score transformation will be used so that all eight SF-12v2® scales have a mean of 50 and a Standard Deviation of 10 in the 1998 US population.
The Euro-Quality of Life (known as Euro-QoL), a generic quality of life instrument, is used to determine health utilities, which are useful in costs evaluations [38]. The Euro-QoL is a brief, self-administered form that evaluates five single-item health dimensions: (1) mobility, (2) self-care, (3) usual activities, (4) pain/discomfort and (5) anxiety/depression, with response options of “no problems, some problems or extreme problems”. The Euro-QoL also includes a visual analog scale (VAS) which is a vertical, graduated (0–100 points) ‘thermometer’, with 100 representing “best imaginable health state” and 0 representing “worst imaginable health state.”
7.0 Cost-effectiveness and Cost-utility
Data collectors use an investigator-developed self-administered questionnaire to collect direct medical costs, direct nonmedical costs, and indirect costs associated with participating in the two study arms over one year. The direct medical costs include costs associated with implementing and maintaining the interventions, side effects of the interventions, and medical care incurred outside of the trial such as the costs of hospitalization, emergency room, urgent care, and outpatient services; telephone calls to health care providers; and prescription medications.
The direct non-medical costs include the value of the time that participants spend traveling to and attending sessions and data collections as well as time spent exercising, shopping, and cooking; the cost of exercise classes and related equipment, food, and food preparation items; and the cost of transportation to and from the sessions and data collections. The indirect costs resulting from lost productivity are those arising from being absent from work or usual activity due to the involvement in the trial, illness and injury. Costs of research other than those related to each intervention will not be included in the cost estimation, as these will be similar in both interventions. For our analysis, we assume that direct medical costs, direct non-medical costs, and indirect costs are not otherwise affected over the period of intervention. We also assume that treatment effectiveness is not changed and that there are no changes in the adherence to the intervention other than that observed.
The main measure of intervention effectiveness will be weight change (main trial outcome) at 12-weeks post intervention. In the cost-effectiveness analysis, we will use health-related quality of life (HRQoL) as the secondary trial outcome. We will perform cost-effectiveness analysis by applying incremental cost estimates from the health system and societal perspectives to the number of kilograms of body weight loss, over a 12-month time horizon. No discounting will be necessary as the intervention only spans 12 months. We will also perform cost-utility analysis by comparing costs to outcomes expressed in units of Health Related Quality of Life (HRQoL) [assessed using the Euro-QoL and the SF-12v2®]. Investigators measure the HRQoL or health utility on a continuum with perfect health receiving a value of 1.0 and health judged equivalent to death a value of 0.0. We will use the direct medical costs and direct nonmedical costs to estimate the cost per unit of HRQoL. As per the recommendation of the Panel on Cost-Effectiveness in Health in Medicine, the analysis from the perspective of a health system will include only direct medical costs [39]. The analysis from the perspective of society will include direct medical costs, direct non-medical costs, and indirect costs
8.0 Sample Size and Power
To determine sample size the statistician based calculations on the primary outcome of weight change and the secondary outcome of FPG. For sample size calculations, the secondary outcome is a mean reduction in FPG levels of at least 3 mg/dl. Although the original DPP study used a 6 mg/dl reduction in FPG, we chose a 3 mg/dl reduction because we assumed that research goals designed for a clinical setting are not easily achievable in a community setting. In addition, due to our attempts to recruit a sample representative of the target population, we relied on diabetes risk factors other than impaired FPG, such as age, race, family history of diabetes, and BMI 25 to determine high-risk status. We determined study outcomes by questionnaires and biological measurements at three times during the study period (baseline, 12–14 weeks post-baseline and 12-months post- baseline).
To achieve the target sample size of 760 participants, we aimed to recruit 40 participants at each of the 20 churches. This sample size provides at least 80% power to detect a 7% reduction in weight and a mean 3 mg/dl reduction in FPG with a level of significance at 5% (one-sided), adjustment for repeated measure comparisons of the two arms, a 0.005 intracluster correlation (ICC), and 20% attrition rate. After adjusting for individual and cluster-level characteristics, we used the median ICC (0.005) as reported by Adams and colleagues [40].
To assess the assumptions used in the sample size determination, in December 2011, we conducted a blinded interim analysis. There were 415 enrolled participants with week-12 data. The analysis revealed statistically significant group differences in the primary outcome measure. Moreover, we noted a substantially lower variability than what we had originally assumed. We also noted an attrition rate of 6.8% opposed to the anticipated 20%. We are therefore confident that the current number of enrolled subjects (n = 604) will provide adequate power to detect statistically meaningful group differences.
9.0 Description of Study Participants
Table 4 presents baseline characteristics of the 604 enrolled participants. Most study participants (n= 504) are female (83.4%), and the mean age is 46.5 years old ± 10.88. Fifty-two percent of participants are married and 75% are employed full-time. We used education as a proxy for income and approximately 84% of the participants have at least some college. These data are consistent with reports that most AA churchgoers are female and middle class [41].
Table 4.
Baseline Characteristics of Enrolled Participants.
| Part a. Qualitative Variables | FBAS | Health Education | Overall | p-value* | ||||
|---|---|---|---|---|---|---|---|---|
| n = 317 | n = 287 | n = 604 | ||||||
|
| ||||||||
| n | % | n | % | n | % | |||
| Female | 267 | 84.2 | 237 | 82.6 | 504 | 83.4 | ||
|
| ||||||||
| Education | Some High School or Less | 10 | 3.2 | 9 | 3.1 | 19 | 3.1 | |
| High School Diploma | 40 | 12.6 | 41 | 14.3 | 81 | 13.4 | ||
| Some College | 112 | 35.3 | 85 | 29.6 | 197 | 32.6 | ||
| College Degree | 70 | 22.1 | 70 | 24.4 | 140 | 23.2 | ||
| Some Graduate or | ||||||||
| Professional School | 17 | 5.4 | 22 | 7.7 | 39 | 6.5 | ||
| Graduate or Professional Degree | 68 | 21.5 | 60 | 20.9 | 128 | 21.2 | .635 | |
|
| ||||||||
| Marital Status | Married | 162 | 51.4 | 150 | 53.0 | 312 | 52.2 | |
| Single | 69 | 21.9 | 64 | 22.6 | 133 | 22.2 | ||
| Widowed | 9 | 2.9 | 10 | 3.5 | 19 | 3.2 | ||
| Divorced | 68 | 21.6 | 47 | 16.6 | 115 | 19.2 | ||
| Other | 7 | 2.2 | 12 | 4.2 | 19 | 3.2 | .386 | |
|
| ||||||||
| Employment Status | Unemployed | 50 | 16.2 | 37 | 13.2 | 87 | 14.8 | |
| Disabled | 10 | 3.2 | 8 | 2.8 | 18 | 3.1 | ||
| Full-Time | 223 | 72.4 | 219 | 77.9 | 442 | 75.0 | ||
| Part-Time | 25 | 8.1 | 17 | 6.0 | 42 | 7.1 | .477 | |
|
| ||||||||
| Parent with Diabetes | Yes | 121 | 38.2 | 123 | 42.9 | 244 | 40.4 | |
| No | 196 | 61.8 | 164 | 57.1 | 360 | 59.6 | .241 | |
|
| ||||||||
| History of Gestational Diabetes | Yes | 12 | 3.8 | 19 | 6.6 | 31 | 5.1 | |
| No or N/A | 305 | 96.2 | 268 | 93.4 | 573 | 94.9 | .115 | |
| Part b. Quantitative Variables | FBAS | Health Education | Overall | p-value** | |||
|---|---|---|---|---|---|---|---|
| n = 317 | n = 287 | n = 604 | |||||
|
| |||||||
| Mean | SD | Mean | SD | Mean | SD | ||
| Age (years) | 46.6 | 10.913 | 46.4 | 10.873 | 46.5 | 10.885 | .825 |
| Weight (kg) | 98.4 | 21.021 | 99.0 | 22.117 | 98.7 | 21.532 | .751 |
| BMI (kg/m2) | 35.8 | 7.023 | 35.7 | 7.596 | 35.7 | 7.294 | .828 |
| Waist Circumference (cm) | 107.8 | 14.955 | 106.8 | 15.913 | 107.3 | 15.413 | .406 |
| A1C | 5.8 | .478 | 5.8 | .482 | 5.8 | .480 | .330 |
| Fasting Plasma Glucose (mg/dl) | 90.1 | 9.967 | 89.9 | 9.403 | 90.0 | 9.696 | .812 |
Chi-square test of homogeneity of Arm proportions.
T-test of equality of Arm means.
Forty percent of study participants have a family history of diabetes. The average participant is within the obese range (BMI 35.7 ± 7.29; weight 98.7 kg ± 21.53; waist circumference 107.3 cm ± 15.41) yet they have a mean glucose value of 90.0 mg/dl ± 9.69 and HbA1C (A1C) value of 5.8% ± .48, which is within the normoglycemic range [34]. A Chi-square test of homogeneity of proportions and a T-test of equality reveal no statistical difference between study arms.
10.0 Statistical Procedures
This cluster-randomized controlled trial includes repeated measures at multiple time points over 12 months, as well as clustering of congregants/participants who are nested within churches (the clusters), with the congregation size acting as a blocking factor. The study design is therefore hierarchical or nested in nature. Consequently, statistical modeling will account for the hierarchical random effects and the repeated outcome measurements. Our major focus in modeling and hypothesis testing will be on group (FBAS intervention vs. Health Education Comparison) differences in weight improvement over time. We will test hypotheses using Generalized Linear Mixed Models (GLIMMs) as implemented in SAS’s GLIMMIX procedure (version 9, SAS Institute, Cary, NC). We will separately model and test hypotheses for each outcome. All hypotheses were planned a priori, thus we will not make any multiple comparison adjustments to the alpha level (Type I error rate). We will use the Bonferroni-Sidak adjustment to conduct post-hoc comparisons. We will conduct all analyses using a 5% one-sided significance level, with the primary hypothesis testing for a significantly greater improvement in the FBAS arm than in the comparison.
The primary outcome (weight) and secondary outcome repeated measures at three time points are: (1) Weight (WT) - Repeated Measures (RM); (2) Fasting Plasma Glucose Level (FPG) - RM; and, (3) Physical Activity (PA) – RM, (4) Hemoglobin A1C (A1C) – RM; (5) Waist Circumference (WC) – RM; Blood Pressure (BP) – RM; (6) Quality of Life Measures (QOL) – RM; and (7) Cost-effectiveness (CE) – RM. The main design factors include: (1) Between Subject Factor - Group (FBAS intervention vs. Comparison); (2) Within-Subject Factor - Time (baseline, 12 weeks post baseline and 12 months post baseline); and, (3) Cluster (Church).
The instrumental/contextual variables (covariates or blocking factors) used in the analyses are: (1) congregation size, (2) participant gender, (3) age, (4) pre-diabetic status, and: (5) overweight status. Selected interactions of particular importance are: (1) group by congregation size; and, (2) pre-diabetic status by over-weight status. Additionally the number of sessions attended and the interaction of attendance with Group will be included in the statistical analyses to adjust for its effect, and to determine whether attendance is a confounder or an effect-modifier. These analyses will allow us to determine if there was an overall dose-response and/or a differential Group dose-response.
We will report appropriate adjusted treatment means (and standard deviations) and ICCs for all analyses and outcome variables. This will help inform power and sample size calculations for future similar studies. We will use the IBM SPSS statistical software for descriptive analyses and SAS for detailed analyses. The principal investigator will remain blinded to church assignment for the intervention and to study outcomes until study completion.
Discussion
To our knowledge, this study is the first to include a sizeable number of AAs in a community-based, randomized control DPP translational program. The success of obtaining a large sample size is due to several factors. We collaborated early on with the community advisory board and study specific advisory group in determining the priority health problems of the target population, socio-cultural preferences, recruitment strategies, and intervention delivery formats. Strength of the study design is that instead of a control condition; we developed a comparison intervention from which participants could potentially benefit and which addressed health issues relevant to the target population [42]. Comparison CHAs delivered the intervention in the same dose and intensity as the FBAS intervention. Therefore all study participants received useful health information, equal attention and intervention dose. Potential participants were aware that their church had a 50% chance of receiving either intervention, yet we feel that our efforts to make the comparison intervention relevant enhanced the participant’s acceptance of randomization and may decrease the threat of differential attrition.
The study design enhanced participation by holding all sessions and evaluations at the church at times agreed upon by the pastors and the research team. Had the interventions been undertaken at a common site significant travel may have been required for each participant and optimal recruitment may have been hampered. Despite the travel burden imposed upon the research team, the most logical approach to carrying out the project was to conduct all aspects at the participants’ individual church.
Having an investigator who is a well-known and trusted health leader in the faith community to help recruit the participating pastors, CHAs and participants facilitated our recruitment process as well as study implementation. The investigator was knowledgeable and sensitive to the AA-church environment and advised the study team on the AA-church culture and norms. This information allowed us to integrate the multilevel intervention into the context of routine church practices rather than require the church to modify their practices in order to accommodate the research study.
There were methodological alternatives to how we conducted this trial. We could have randomized participants rather than churches, but the logistics of individuals going to churches other than their own, perhaps far from their homes would have been burdensome, and potentially would have negatively affected recruitment and session attendance. Alternatively, delivering both interventions at an individual church could have led to contamination of both groups.
The use of health professionals from within the churches for intervention delivery was a unique aspect of FBAS. Although other researchers have engaged members of the target population to reach research participants and to deliver interventions, most have trained lay community workers rather than health professionals and have used unstructured curriculum. Cited weaknesses of this approach are inconsistent personnel and an unstructured, eclectic intervention delivery [21]. FBAS is the only diabetes-prevention translation study to our knowledge that has relied solely upon the churches’ trained health-ministry members to deliver a structured-DPP translational intervention. According to Ali and colleagues, participant weight change among community DPP interventions is similar across trials regardless of the educational level of the person delivering the intervention [12]. However, from our experience, the professional background of health ministry members enables them to master health intervention delivery easily. Furthermore, their commitment and membership stability to an individual congregation makes them accessible to researchers for additional training. Consequently, they may be ideal partners to address long-term public health matters in this population.
Interventions aimed at preventing the onset of diabetes among AA congregants require an operational definition of high-risk status. Often, researchers use the level of FPG or more recently A1C level to define diabetes risk status [34]. While there is considerable discussion in the literature as to when the risk for diabetes begins, defining diabetes risk with factors other than glucose status, such as family history of diabetes and weight status may facilitate community-based implementation of diabetes prevention interventions and improve external validity [43].
Another unique feature of FBAS compared with other faith-based DPPs is the collection of data for cost utility analysis. Cost-effective community models for successful DPP translation interventions will help inform decisions regarding the feasibility of widespread dissemination as well as sustainability of the interventions.
Although this trial may hold promise for dissemination, it is not without potential limitations. One investigator who was well known and trusted by the target population played a key role in church recruitment; the investigator could have introduced selection bias. We attempted to control for this potential limitation by making concerted efforts to recruit churches using several different methods, such as contacting churches that CAB members identified and an in-person presentation at the local ministers meeting. The setting of the study, the faith-based approach, the middle-class and predominately AA women participant sample, the congregation size of more than 200 members, and our use of incentives to foster and sustain participation limit the generalizability of our findings.
Finally, the use of monetary incentives for general dissemination and sustainability is not likely. Those wanting to implement our findings into their communities may want to consider non-monetary incentives. Significant findings from our study may improve the possibility of resources provided for community-based DPP translation programs including those conducted within churches.
In conclusion, although this study has potential limitations, it may also hold great promise for DPP dissemination. FBAS has facilitated interest in diabetes prevention among AA adults. If effective, the study design and implementation have important implications for cost-effective diabetes prevention in faith-based settings.
Acknowledgments
Role of funding source
Funded by the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, grant R18DK082401. The sponsor did not have a role in the design; collection, analysis, or interpretation of data; writing of the paper; or the decision to submit the paper for publication.
We are indebted to Jeannette Andrews, PhD, RN and the Georgia Health Sciences University Community Advisory Board for identifying the need for community-based research related to obesity and diabetes among AAs. We acknowledge the Diabetes Prevention Support Center of the University of Pittsburgh for training and support in the Group Lifestyle Balance program; the FBAS program is a derivative of this material. We appreciate the support of the pastors, congregations, Church Health Advisors, and GHSU volunteer data collectors for their role in project implementation. We also appreciate the pastors and CHAs who reviewed the manuscript prior to submission. We also acknowledge Ken Resnicow, PhD for motivational-interviewing consultations and Sunita Dodani, MD, for her initial contributions to the study. We are also indebted to our research assistants Kamilah Freeman and Lauren Morris for their commitment to all aspects of study implementation.
Abbreviations
- AA
African-Americans
- FBAS
Fit Body and Soul
- DPP
Diabetes Prevention Program
- GLB
Group Lifestyle Balance
- CHA
Church Health Advisor
- T2DM
Type 2 Diabetes Mellitus
- WC
Waist Circumference
- CAB
Community Advisory Board
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Lovoria B. Williams, Email: LWilliams@georgiahealth.edu.
Richard W. Sattin, Email: RSattin@georgiahealth.edu.
James Dias, Email: JDias@georgiahealth.edu.
Jane T. Garvin, Email: BGarvin@georgiahealth.edu.
Lucy Marion, Email: LuMarion@georgiahealth.edu.
Thomas Joshua, Email: TJoshua@georgiahealth.edu.
Andrea Kriska, Email: KriskaA@edc.pitt.edu.
M. Kaye Kramer, Email: Kramerk@edc.pitt.edu.
Justin B. Echouffo-Tcheugui, Email: jechouf@emory.edu.
Arin Freeman, Email: arinfreeman@gmail.com.
K.M. Venkat Narayan, Email: KNaraya@emory.edu.
References
- 1.Centers for Disease Control and Prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. U.S. Department of Health and Human Services; Atlanta, GA: Centers for Disease Control and Prevention; [Google Scholar]
- 2.Mokdad A, Ford ES, Bowman, Deitz WH, Vinicor F, Bales BS, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors 2001. JAMA. 2003;289(1):76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 3.Diabetes Prevention Program Research Group. Reduction in the incidence of Type 2 DM with lifestyle intervention or Metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ackermann RT, Marrero DG. Adapting the diabetes prevention program lifestyle intervention for delivery in the community: The YMCA model. Diabetes Edu. 2008;33(69):69–78. doi: 10.1177/0145721706297743. [DOI] [PubMed] [Google Scholar]
- 5.Katula JA, Vitolins MZ, Rosenberger E, Blackwell C, Morgan T, Lawlor M, et al. One-Year Results of a Community-Based Translation of the Diabetes Prevention Program: Healthy-Living Partnerships to Prevent Diabetes (HELP PD) Project. Diabetes Care. 2011;34(7):1451–1457. doi: 10.2337/dc10-2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seidel MC, Powell RO, Zgibor JC, Siminerio LM, Piatt GA. Translating the Diabetes Prevention Program into an urban medically underserved community: a nonrandomized prospective intervention study. Diabetes Care. 2008;31(4):684–9. doi: 10.2337/dc07-1869. [DOI] [PubMed] [Google Scholar]
- 7.Kramer M, Kriska AM, Venditti EM, Semler L, Miller R, McDonald T, et al. A novel approach to diabetes prevention: Evaluation of the Group Lifestyle Balance program delivered via DVD. Diabetes Research and Clinical Practice. 2010;90(3):e60–e63. doi: 10.1016/j.diabres.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 8.Kramer MK, McWilliams JR, Chen, Siminerio LM. A Community-Based Diabetes Prevention Program: Evaluation of the Group Lifestyle Balance Program Delivered by Diabetes Educators. Diabetes Educ. 2011;37(5):659–68. doi: 10.1177/0145721711411930. [DOI] [PubMed] [Google Scholar]
- 9.Kramer M, Venditti EM, Semler LN, Kriska A, Miller R, Orchard T. Long-term Strategies for Diabetes Prevention: Evaluation of the Group Lifestyle Balance Post-Core Sessions Focusing on Carbohydrate and Hunger Management. [last accessed October 27, 2012];J of Diab Metab. 2012 http://www.omicsonline.org/2155-6156/2155-6156-S2-006.php?aid=4819.
- 10.Center for Disease Control and Prevention. [last accessed August 29, 2012];Diabetes is common, disabling, deadly and on the rise. 2009 from http://www.cdc.gov/Features/dsDiabetesTrends.
- 11.Flegal K, Carroll MD, Kit B, Ogden CL. Prevalence of obesity trends in the distribution of body mass index among us adults 1999–2010. JAMA. 2012;307(5):491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 12.Ali MK, Echouffo-Tcheugui JB, Williamson D. How effective were lifestyle interventions in real-world settings that were modeled on the diabetes prevention program? Health Aff. 2012;31(1):67–75. doi: 10.1377/hlthaff.2011.1009. [DOI] [PubMed] [Google Scholar]
- 13.Markens S, Fox SA, Taub B, Gilbert M. Role of Black churches in health promotion programs: lessons from the Los Angeles Mammography Promotion in Churches Program. Am J Public Health. 2002;92:805–810. doi: 10.2105/ajph.92.5.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baskin ML, Resnicow K, Campbell MK. Conducting health interventions in black churches: a model for building effective partnerships. Ethn Dis. 2001;11:822–833. [PubMed] [Google Scholar]
- 15.Carter-Edwards L, Jallay YB, Goldmon MV, Roberson JT, Hoyo C. Key attributes of health ministries in African American churches: an exploratory survey. N C Med J. 2006;67(5):345–350. [PubMed] [Google Scholar]
- 16.Lasater TM, Becker DM, Hill MN, Gans KM. Synthesis of findings and issues from religious-based cardiovascular disease prevention trials. Ann Epidemio. 1997;7(7):S46–S53. [Google Scholar]
- 17.Dodani S, Kramer K, Williams L, Crawford S, Kriska A. Fit Body & Soul: A church-based behavioral lifestyle program for diabetes prevention in African-Americans. Ethn Dis. 2009;19:135–141. [PubMed] [Google Scholar]
- 18.Davis-Smith M. Implementing a diabetes prevention program in a rural African- American church. J Natl Med Assoc. 2007;99(4):440–445. [PMC free article] [PubMed] [Google Scholar]
- 19.Yeary KH, Cornell C, Turner J, Moore P, Bursac Z, Prewitt TE, et al. Feasibility of an evidence-based weight loss intervention for a faith-based, rural, African American population. Prev Chronic Dis. 2011;8(6):A146. [PMC free article] [PubMed] [Google Scholar]
- 20.Boltri J, Davis-Smith M, Okosun, Seale JP, Foster B. Translation of the National Institutes of Health Diabetes Prevention Program in African American churches. J Natl Med Assoc. 2011;103(3):194–202. doi: 10.1016/s0027-9684(15)30301-1. [DOI] [PubMed] [Google Scholar]
- 21.Faridi Z, Shuval K, Njike V, Katz JA, Jennings G, Williams M, et al. Partners reducing effects of diabetes (PREDICT): a diabetes prevention physical activity and dietary intervention through African-American churches. Health Educ Res. 2010;25(2):306–315. doi: 10.1093/her/cyp005. [DOI] [PubMed] [Google Scholar]
- 22.Wallerstein N, Duran B. Using community-based participatory research to address health disparities. Health Promot Pract. 2006;7:312–323. doi: 10.1177/1524839906289376. [DOI] [PubMed] [Google Scholar]
- 23.McTigue KM, Conroy MB, Bigi L, Murphy C, McNeil M. Weight loss through living well: translating an effective lifestyle intervention into clinical practice. Diabetes Educ. 2009;35 (20):199–204. doi: 10.1177/0145721709332815. [DOI] [PubMed] [Google Scholar]
- 24.University of Pittsburgh. [last accessed January 2, 2013];Diabetes Prevention Support Center. http://www.diabetesprevention.pitt.edu/about.aspx.
- 25.Resnicow K, Kramish Campbell M, Carr C, McCarty F, Wang T, Periasamy S, et al. Body and soul: A dietary intervention conducted through African-American churches. Am J Prev Med. 2004;27(2):97–105. doi: 10.1016/j.amepre.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 26.Stokols D. Translating social ecological theory into guidelines for community health promotion. Am J Health Promot. 1996;10(4):282–298. doi: 10.4278/0890-1171-10.4.282. [DOI] [PubMed] [Google Scholar]
- 27.Dodani S, Fields JZ. Implementation of the Fit Body and Soul, a church-based life style program for diabetes prevention in high-risk African Americans. The Diabetes Educ. 2010;36(3):465–472. doi: 10.1177/0145721710366756. [DOI] [PubMed] [Google Scholar]
- 28.Kramer MK, Kriska A, Venditti E, Miller RG, Brooks M, Burke L, et al. Translating the Diabetes Prevention Program: A Comprehensive Model for Prevention Training and Program Delivery. Am J Prev Med. 2009;37(6):505–511. doi: 10.1016/j.amepre.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 29.Center for Disease Control. [last accessed October 27, 2012];The Community Guide to Preventive Services. http://www.thecommunityguide.org/index.html.
- 30.Diabetes Prevention Program Research Group. The Diabetes Prevention Program. Design and methods for a clinical trial in the prevention of type 2 diabetes. Diabetes Care. 1999;22(4):623–34. doi: 10.2337/diacare.22.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Diabetes Prevention Program Research Group. The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care. 2002;25(12):2165–2171. doi: 10.2337/diacare.25.12.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. [last accessed January 6, 2013];Motivational Interviewing. http://www.motivationalinterview.org/index.html.
- 33.Shephard RJ. Canadian Home Fitness Test and exercise screening alternatives. Sports Med. 1988;5:185–195. doi: 10.2165/00007256-198805030-00005. [DOI] [PubMed] [Google Scholar]
- 34.American Diabetes Association. Standards of medical care in diabetes--2010. Diabetes Care. 2010;33 (Suppl 1):S11–61. doi: 10.2337/dc10-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim C, Bullard K, Herman W, Beckles G. Association between Iron Deficiency and A1C levels among adults without diabetes in the National Health and Nutrition Examination Survey, 1999–2006. Diabetes Care. 2010;33(4):780–785. doi: 10.2337/dc09-0836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med & Sci Sports Exerc. 2003;35(8):1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 37.Ware JE, Jr, Kosinski M, Turner-Bowker DM, Gandek B. How to Score Version 2 of the SF-12v2® Health Survey (With a Supplement Documenting SF-12® Health Survey) Lincoln, RI: Quality Metric Incorporated; 2002. [Google Scholar]
- 38.EuroQOL Group. Euro-QOL: A new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 39.Russell LB, Gold, Siegel JE, Daniels N, Weinstein MC. The Role of Cost-effectiveness Analysis in Health Medicine. JAMA. 1996;276 (14):1172–1177. [PubMed] [Google Scholar]
- 40.Adams G, Gulliford MC, Ukoumunne OC, Eldridge S, Chinn S, Campbell MJ. Patterns of intra-cluster correlation from primary care research to inform study design analysis. J Clin Epidemiol. 2004;57(8):785–94. doi: 10.1016/j.jclinepi.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 41.Pew Forum on Religion and Public Life. [last accessed October 9, 2012];A religious portrait of African-Americans. http://www.pewforum.org/A-Religious-Portrait-of-African-Americans.aspx.
- 42.Melynyk BM, Morrison-Beedy D, Moore SM. Nuts and bolts of intervention design. In: Melynyk BM, Morrison-Beedy D, editors. Intervention research: Designing, conducting, analyzing, and funding. New York: Springer Publishing Company; 2012. pp. 37–52. [Google Scholar]
- 43.Echouffo-Tcheugui JB, Ali MK, Griffin SJ, Narayan KM. Screening for Type 2 Diabetes and Dysglycemia. Epidemiol Rev. 2011;33 (1):63–87. doi: 10.1093/epirev/mxq020. [DOI] [PubMed] [Google Scholar]