Table 2.
Scope of the reaction
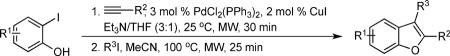
| entry | iodophenol | acetylene | aryl iodide | product | yielda (%) |
|---|---|---|---|---|---|
| 1 |
|
5 | 6 |
|
91 |
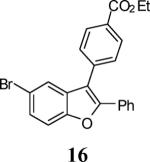
| 2 |
|
5 | 6 |
|
92 |
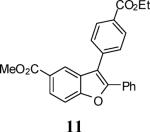
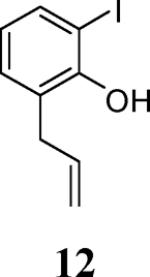
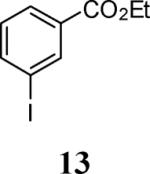
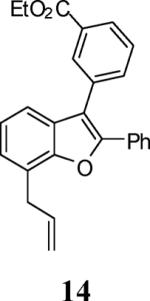
| 3 |
|
5 |
|
|
53 |
| 4 |
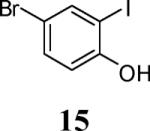
|
5 | 6 |
|
84 |
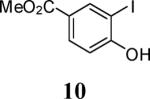
| 5 | 15 | 5 | 13 |
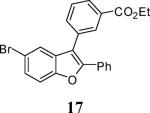
|
60b |
| 6 | 4 |
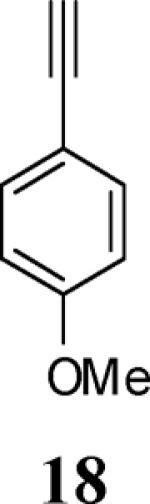
|
6 |
|
94 |
| 7 | 4 |
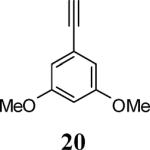
|
6 |
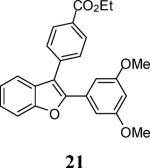
|
93 |
| 8 | 4 |
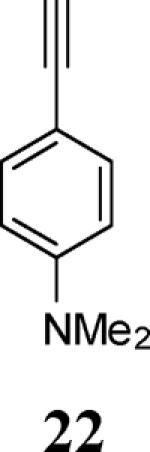
|
6 |
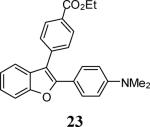
|
83 |
| 9 | 4 |
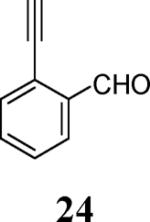
|
13 |
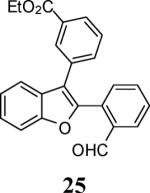
|
69c |
| 10 | 4 |
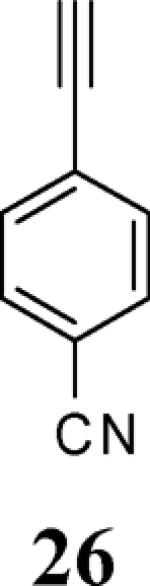
|
6 |
|
traced |
| 11 | 10 |
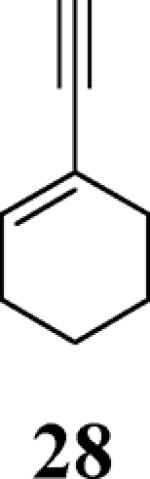
|
13 |
|
52 |
| 12 | 4 |
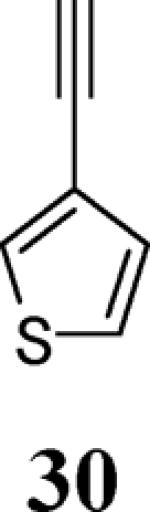
|
6 |
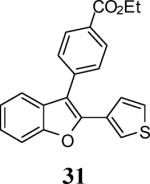
|
100 |
| 13 | 4 |
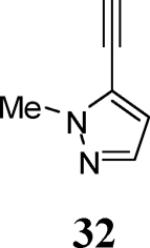
|
6 |
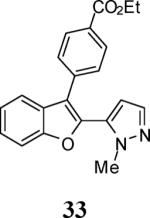
|
63e |
| 14 | 4 | 5 | PhI |
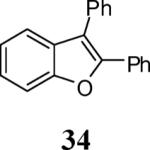
|
87 |
| 15 | 4 | 5 |
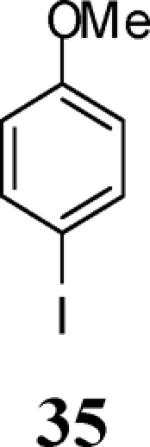
|
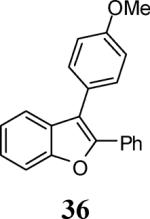
|
53f |
| 16 | 4 | 5 |
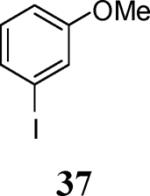
|
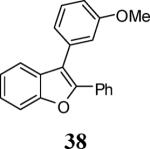
|
84 |
| 17 | 4 | 5 |
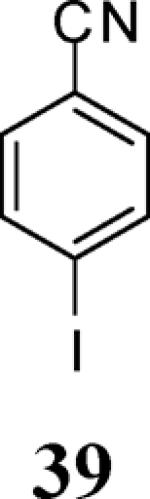
|
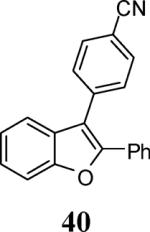
|
98 |
| 18 | 4 | 5 |
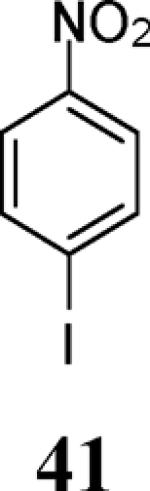
|
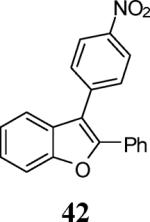
|
75 |
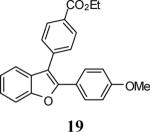
| 19 | 4 | 5 |
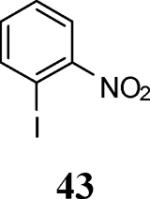
|
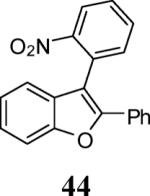
|
74 |
| 20 | 4 | 5 |
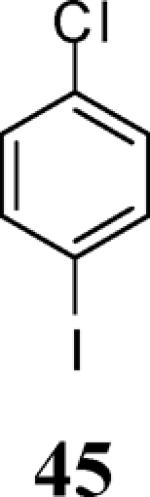
|
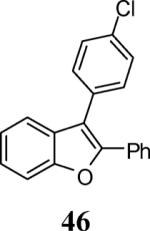
|
96 |
| 21 | 4 | 30 |
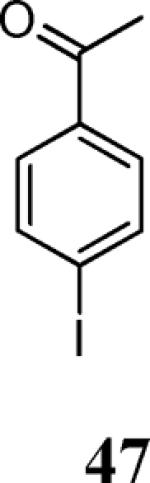
|
|
73 |
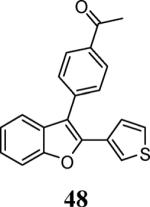
| 22 | 4 | 5 |
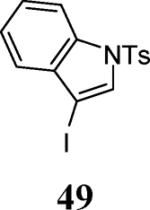
|
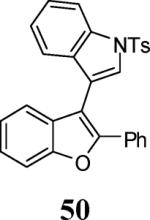
|
58 |
| 23 | 4 | 5 |
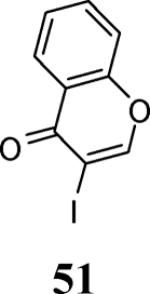
|
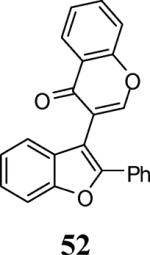
|
43 |
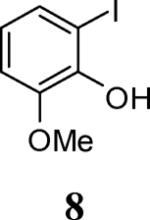
| 24 | 8 |
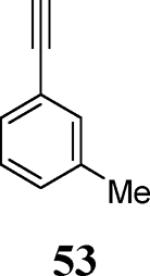
|
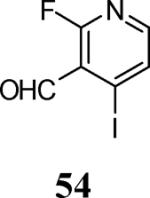
|
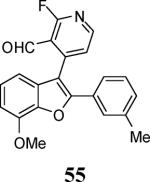
|
65 |
| 25 |
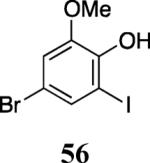
|
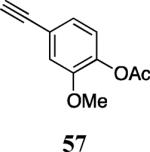
|
|
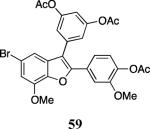
|
60 |
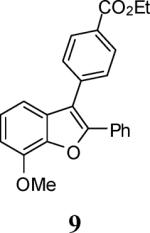
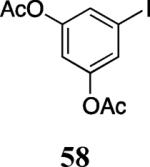
| 26 |
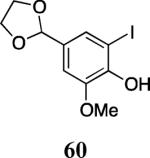
|
57 | 58 |
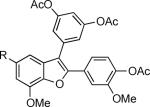
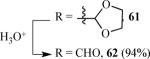
|
63 |
| 27 | 4 | 5 |
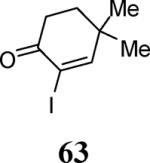
|
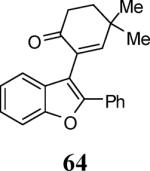
|
34 |
Isolated yields after column chromatography.
This compound was prepared on a large scale and recrystallized, what might have contributed to the lower yield.
1.0 Equiv of alkyne was employed.
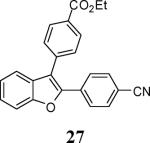
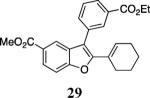
The reaction afforded a complex mixture; for an alternative route to 27, see Scheme 2.
1.0 Equiv of alkyne was employed and the first step of the process was run at 60 °C.
The second step of the process was conducted at 80 °C with the addition of 10 mol % Pd(PPh3)4.
