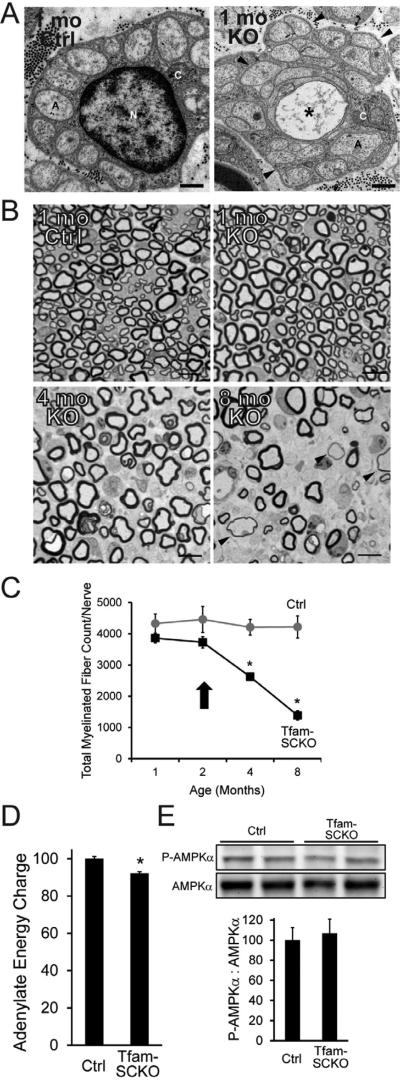
Figure 1. SC mitochondrial dysfunction induces a progressive, degenerative peripheral neuropathy that is not directly linked to energy depletion.
(A) Electron micrographs of 1-month-old Ctrl and Tfam-SCKO sciatic nerve cross sections depicting early structural abnormalities of Remak bundles (SC surrounding multiple unmyelinated axons; arrowheads) and degeneration of unmyelinated axons (asterisk). A, axon; N, SC nucleus; C, SC cytoplasm. Scale bar 500 nm. (B, C) Toluidene blue stained plastic sections of Tfam-SCKO and Ctrl sciatic nerve cross sections (B) and quantification of total number of myelinated profiles per nerve (C) at different ages show prominent, progressive degeneration of large-caliber myelinated axons and demyelination starting at 3-4 months of age. Arrowheads (B) indicate axons surrounded by unusually thin myelin, a sign of demyelination. Arrow (C) indicates the point in the progression of the pathology for all mice used in later experiments; note that at this age nerves display only limited, early pathological changes with minimal axon loss and demyelination. N=4 mice per genotype at each age. *P<0.01 Scale bar 25 μm. (D) Adenylate energy charge in 2-month-old Tfam-SCKO nerves shows only a slight decrease in the energy levels of Tfam-deficient SCs compared to Ctrl nerves. N=8 mice per genotype. *P<0.01 (E) Immunoblot analysis and quantification of band intensity reveals no increase in the phosphorylation (activation) of the energy sensor AMPK in 2-month-old Tfam-SCKO nerves, indicating that energy depletion is an unlikely driver of nerve pathology in these mice. N=4 mice per genotype.