Figure 6.
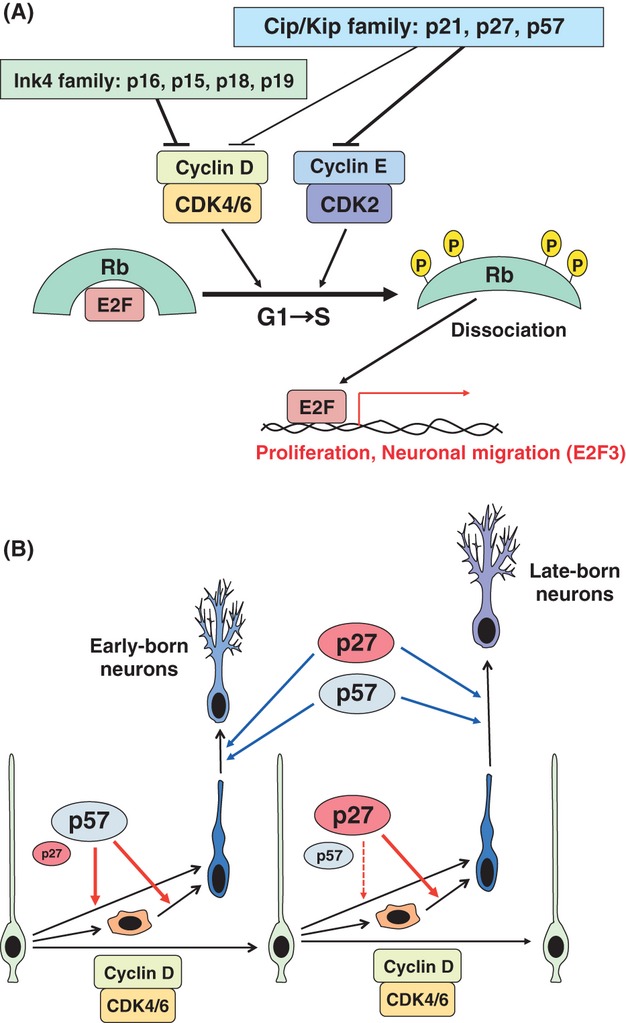
Cyclin-dependent kinase (CDK) inhibitor proteins regulate cell cycle progression, growth arrest, and postmitotic neuronal migration. (A) Molecular mechanisms for G1/S transition. The transition from G1 to S phase is dependent on CyclinD-Cdk4/6 and CyclinE-Cdk2 activities, which phosphorylate Rb protein. The phosphorylated Rb protein dissociates E2F family transcription factors. Both E2F1 and E2F3 promote G1/S transition in neural progenitors, whereas E2F3, but not E2F1, regulates neuronal positioning. The activities of Cyclin-CDK complexes are suppressed by CDK inhibitor proteins, which are composed of a Cip/Kip family (p21cip1, p27kip1, and p57kip2) and Ink4 family (p16Ink4a, p15Ink4b, p18Ink4c, and p19Ink4d). (B) Roles of CDK inhibitor proteins, p27kip1 and p57kip2, in cell cycle exit and subsequent neuronal migration. p57kip2 and p27kip1 preferentially control the cell cycle exit of neural progenitors for early-born (deep layer) and late-born (upper layer) neurons, respectively. p27kip1 mainly functions in basal progenitors (orange cells) rather than apical progenitors (green cells). Both p27kip1 and p57kip2 have been shown to regulate the migration of postmitotic neurons as well as the cell cycle exit.
