Figure 9.
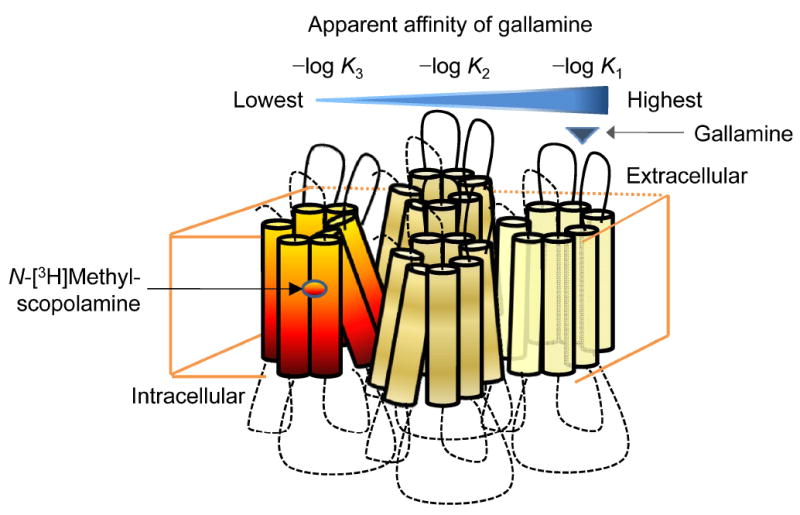
Heterogeneity induced by N-[3H]methylscopolamine among the allosteric sites of an oligomer. Binding of [3H]NMS to a tetramer configured as a square or a rhombus gives rise to a conformational asymmetry characterized by three classes of protomer: those occupied by [3H]NMS (red–yellow), those adjacent to protomers occupied by [3H]NMS (yellow), and those diagonally opposite protomers occupied by [3H]NMS (light yellow). Gallamine binds with highest apparent affinity to the allosteric site of an otherwise vacant protomer that is most distant from a single protomer occupied by [3H]NMS (i.e., log K1). It binds with lowest apparent affinity to the allosteric site of a protomer in which the local orthosteric site is occupied by [3H]NMS (i.e., log K3).
