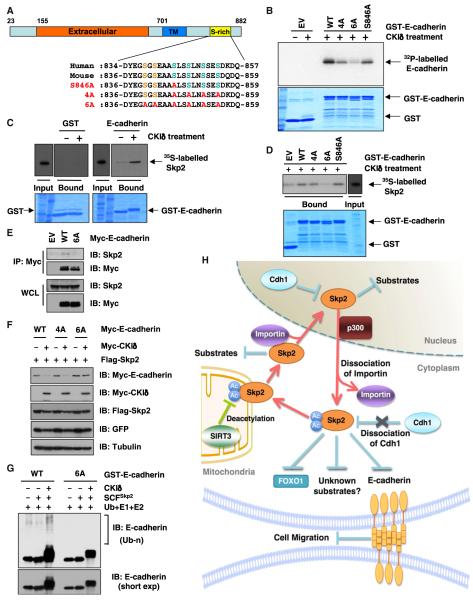
Figure 7. Skp2 Promotes the Ubiquitination and Destruction of E-Cadherin in a CKI-Dependent Manner.
(A) Sequence alignment of the putative CKI phosphorylation sites in Skp2 from various species.
(B) Indicated GST-fusion proteins were incubated with CKIδ and [γ-32P]ATP. The kinase reaction products were resolved by SDS-PAGE, and phosphorylation was detected by autoradiography.
(C and D) Autoradiography of 35S-labeled Skp2 bound to the indicated GST-fusion proteins. Where indicated, GST-fusion proteins were pretreated with CKId before pull-down assays were performed.
(E) IB analysis of WCL and anti-Myc IP derived from HeLa cells transfected with the indicated Myc-E-cadherin constructs.
(F) IB analysis of WCL from HeLa cells transfected with the indicated plasmids.
(G) SCFSkp2 E3 ligase complex promotes E-cadherin ubiquitination in vitro. Where indicated, GST-E-cadherin proteins were pretreated with CKI before the in vitro ubiquitination assays.
(H) Proposed model for how acetylation of Skp2, which subsequently regulates Skp2 stability and cellular localization to influence its oncogenic functions, is governed by both p300 and SIRT3.
See also Figure S7.