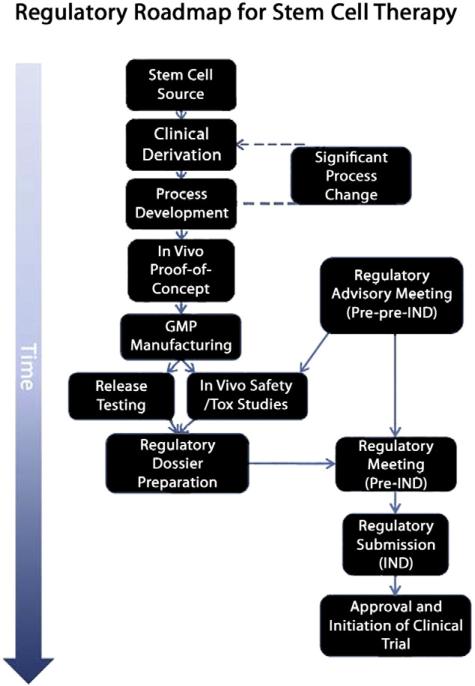
Fig. 3.
Roadmap to the clinic. Clinical derivation of the cell line of interest represents the initial stage of translating a cell therapy to the clinic. The ability to manufacture the stem cell product to current clinical expectations is addressed in process development before the clinical prototype cell is shown to be efficacious in animal models of disease. Note that significant late-stage changes to processes may result in rederivation and repeating key experiments. Based on advisory meetings with regulatory bodies (in this case the US FDA), release testing and safety/toxicology (tox) programs are initiated with the GMP manufactured cell product or equivalent. The regulatory dossier is compiled and further advice sought from the regulatory agency before submitting the Investigational New Drug (IND) application for a phase 1 clinical trial.