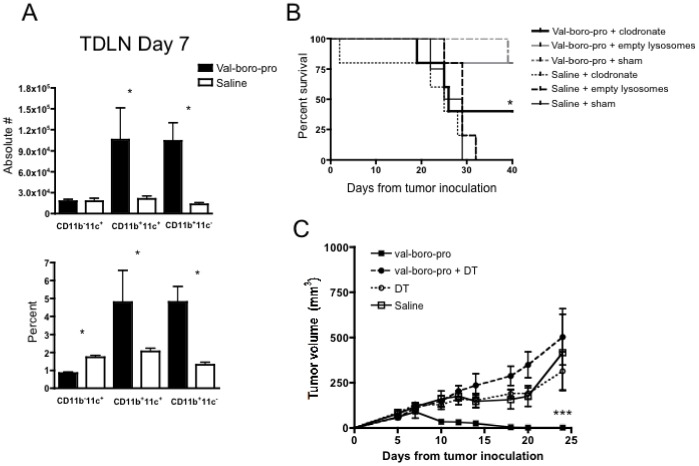
Figure 7. Myeloid dendritic cells (CD11b+CD11c+) are increased in TDLN and are required for tumor regression.
(A) C57BL/6 mice were challenged with 1×106 MB49 on day 0 and treated with Val-boroPro (closed bars) or saline (open bars) during week one (n = 4/group). Tumor-draining lymph nodes were harvested and analyzed by flow cytometry on day 7 (p<0.05, Mann-Whitney) (B) C57BL/6 mice were inoculated with 106 MB49 and injected with clodronate IP (0.1 mL/10 g body weight) every other day from day –1 to 9 following tumor inoculation. Control groups were injected with empty lysosomes or saline (sham). Val-boroPro was administered orally for 1 week (days 3–7). Survival was significantly different in Val-boroPro treated mice given clodronate (thick solid line) compared to those treated with Val-boro-Pro and given empty lysosomes (gray solid line) (*p<0.05, Logrank test, n = 5/group). (C) CD11c-diphtheria toxin (DT) chimeric mice were generated by transplanting bone marrow from CD11c-DT transgenic mice into lethally irradiated C57BL/6 recipients (n = 5/group). Female chimeras were inoculated with 106 MB49 on day 0 and treated with Val-boroPro or saline during week one (days 3–7) with or without IP injections of DT (8 ng/1 g body weight) every other day from day –1 to 9. Tumor volumes were significantly larger in Val-boroPro treated chimeric mice receiving DT (closed circles) compared to Val-boro-Pro treated chimeric mice receiving saline (closed squares) (p<0.001 for all timepoints beyond day 10). Tumor volumes were not statistically different between saline treated (open squares) and DT treated (open circles) chimerics not treated with Val-boroPro. Experiments displayed in Figures 7A–C were each conducted twice.