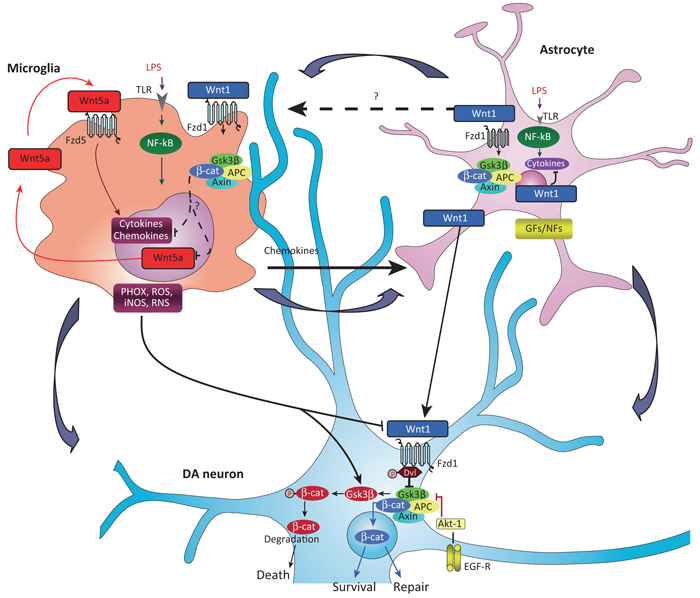
Figure 2.
Wnt tripartite regulation in the brain. Macrophage/microglia harbor Fzd5 and Fzd1 subtype receptors and can respond to both non-canonical Wnt5a- and canonical Wnt1-type ligands [9,18,40]. Upon inflammatory challenge with LPS or brain injury, TLRs and IFN-γ-mediated activation, macrophage/microglia produce a panel of proinflammatory cytokines and chemokines, of which Wnt5a constitutes one part of a self-perpetrating cycle, via autocrine Wnt5A/CamKII activation and paracrine stimulation of Th-1 cytokines [40]. Upregulation of microglial PHOX-derived ROS, iNOS-derived NO, and GSK-3β, a known regulator of NF-κB-dependent gene transcription, further exacerbate microglia reaction [8,10]. Chemokine-activated astrocytes respond to microglia by inducing the expression and release of Wnt1-type ligands [8,10,17]. It is proposed that astrocyte-derived Wnt1-type ligands may restrain inflammation, via stimulation of microglia Fzd1 receptors (in analogy to what is observed in macrophages [40]), possibly resulting in the attenuation of cytokines and Wnt5a overexpression (broken arrows). At the neuronal level, MPTP-induced microglial PHOX and RNS upregulate GSK-3β in DA neurons [8,10,17] leading to β-catenin phosphorylation and proteosomal degradation, which may increase DA neuron vulnerability and further incite cell death [8,17]. However, depending on the severity of brain insult, the degree of inflammation and specific vulnerability factors (age, gender, concomitant presence of stressors and gene mutations [87]), astrocyte–neuron crosstalk, via Wnt1 may serve a protective role, via stabilization of β-catenin in the cytoplasm, its nuclear translocation, followed by transcription of prosurvival genes, that may lead to neuroprotection and/or neurorepair [8,17]. Chemokine-activated astrocytes can also promote neurogenesis from adult VM progenitors, in vitro, via Wnt/β-catenin signaling activation [8]. In stark contrast, aging-induced loss of astrocyte-derived Wnt1 response [8,15], or Wnt/β-catenin signaling antagonism with Dkk1 [17], may result in DA neuron failure to repair. Potential crosstalk between glial GFs/NFs and Wnt/β-catenin signaling via Akt/GSK-3β/β-catenin cascades are also illustrated (see [17]). Abbreviations: CamKII, Ca2+–calmodulin-dependent protein kinase II; DA, dopamine; Dkk1, Dickkopf 1; EGF, epidermal growth factor; Fzd, Frizzled; GFs/NFs, glial-derived growth/neurotrophic factors; IFN-γ, interferon-γ; LPS, lipopolysaccharide; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; NF-κB, nuclear factor (NF)-κB; PHOX, phagocyte oxidase; RNS, reactive nitrite species; ROS, reactive oxygen species; Th-1, T helper 1 cells; TLR, Toll-like receptor; VM, ventral midbrain; Wnt, Wingless-related MMTV integration site.